Do Doses Differentiate Hepatitis B Vaccine and Acute Myocardial Infarction Risk?

Since Hepatitis B vaccination is the most effective strategy for prevention of the hepatitis V virus (HBV) and is a critical component of global hepatitis elimination efforts, the reason behind under-vaccinations is not well known.
Furthermore, HBV is a vaccine-preventable viral disease that continues to cause substantial morbidity and mortality.
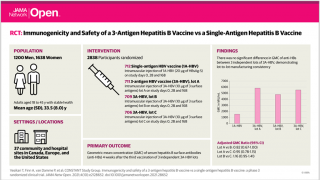
Researchers conducted an Original Investigation in a clinical setting to compare the rate of acute myocardial infarction (MI) between recipients of the HepB-CpG vaccine and HepB-alum vaccine.
They found the receipt of the HepB-CpG vaccine compared with the HepB-alum vaccine was not significantly associated with an increased risk of acute MI.
Fifty-two type 1 acute MI events were confirmed among recipients of HepB-CpG vaccine for a rate of 1.67 per 1000-person-years, and 71 type 1 acute MI events were confirmed among recipients of HepB-alum vaccine for a rate of 1.86 per 1000 person-years (absolute rate difference, −0.19 [95% CI, −0.82 to 0.44]; adjusted HR, 0.92 [1-sided 97.5% CI, ∞ to 1.32], which was below the noninferiority margin; P < .001 for noninferiority).
These findings were published by the JAMA Network on Mar. 25, 2022, and were consistent across subgroups defined by age group, diabetes, hypertension, receipt of concomitant vaccines, and whether the index dose was the first or subsequent hepatitis B vaccine dose.
Similar results from sensitivity analyses using different analytic approaches and outcome definitions and a lack of temporal clustering or a dose-response combine to provide strong evidence that the HepB-CpG vaccine was not associated with acute MI.
Further research is needed to determine if individuals would be more likely to initiate a shorter 2-dose series than a more extended 3-dose series and address vaccine hesitancy and other barriers to hepatitis B vaccine uptake among adults.
A strength of this study was the prospective cohort design in which hepatitis B vaccines were administered as part of routine health care delivery.
Because only one adult hepatitis B vaccine product was available per facility, the study design inherently minimized selection bias associated with the choice of hepatitis B vaccine product based on a patient’s risk profile.
Moreover, the U.S. CDC released updated hepatitis vaccination schedules for children, adolescents, and adults on Feb. 17, 2022.
Other hepatitis vaccine studies are posted at PrecisionVaccinations.com/hepatitis.
Note: This news article edited the study for clarity and was curated for mobile readers. This study was funded by Dynavax Technologies, manufacturers of HEPLISAV-B. And these researchers disclosed various industry relationships.
Our Trust Standards: Medical Advisory Committee