Marburg Disease Vaccine Candidate Advances to Phase 2 Study

The Sabin Vaccine Institute today announced it launched a Phase 2 clinical trial for its vaccine candidate against the Marburg virus disease (MVD).
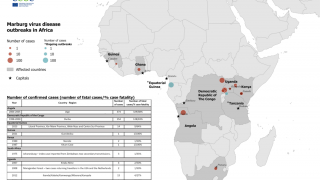
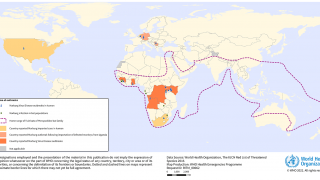
As of October 20, 2023, there are currently no vaccines or antiviral treatments approved to treat Marburg, a filovirus in the same family as the virus that causes Ebola.
Marburg has a case fatality rate of up to 88%.
Based on the ChAd3 platform, Sabin’s single-dose investigational Marburg vaccine was found to be promising in a Phase 1 clinical trial.
Dr. Betty Mwesigwa, deputy executive director of the Makerere University Walter Reed Project, is the principal investigator for the Kampala portion of the Sabin-sponsored trial.
A few weeks later, participants will also be enrolled at a second site at the Kenya Medical Research Institute, with Dr. Videlis Nduba as principal investigator.
In a press release, Amy Finan, Sabin’s Chief Executive Officer, commented, “Sabin’s Phase 2 clinical trial builds on a solid safety and immunogenicity foundation, and we hope it will generate the information needed to move the vaccine toward licensure.”
In addition to the Sabin vaccine candidates, other Marburg vaccines are conducting clinical trials.
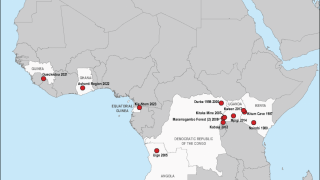
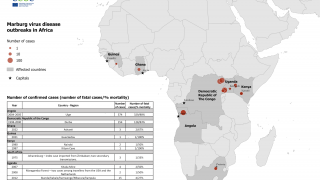
According to Sabin, the number of MVD outbreaks in Africa has climbed steadily in recent years.
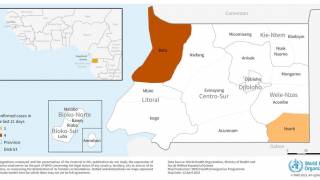
Two outbreaks of Marburg virus disease have occurred in 2023: Equatorial Guinea reported its first documented Marburg outbreak, which killed 12 people, followed by Tanzania, where six people succumbed to the virus.
Communities in Uganda and Kenya are familiar with Marburg, having been ravaged by outbreaks over multiple years in the last few decades.
MVD was first observed in 1967 during outbreaks in Germany.
The U.S. Centers for Disease Control and Prevention published Health Alert Network CDCHAN-00489 on April 6, 2023, confirming no cases of MVD have been reported in the U.S.
Our Trust Standards: Medical Advisory Committee