Heat Stable Marburg Vaccine Candidate Awarded Orphan Drug Designation
Soligenix, Inc. announced today that the Office of Orphan Products Development of the United States Food and Drug Administration (FDA) has granted orphan drug designation to the active ingredient in MarVax™, the subunit protein vaccine of recombinantly expressed Marburg marburgvirus (MARV) glycoprotein, for "the prevention and post-exposure prophylaxis against MARV infection."
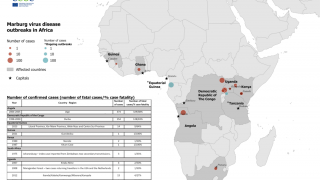
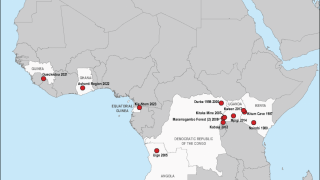
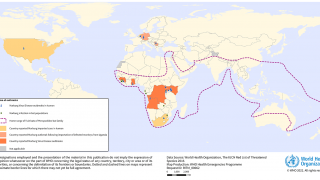
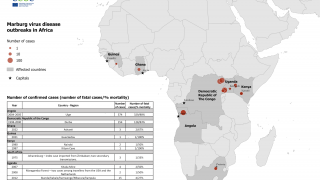
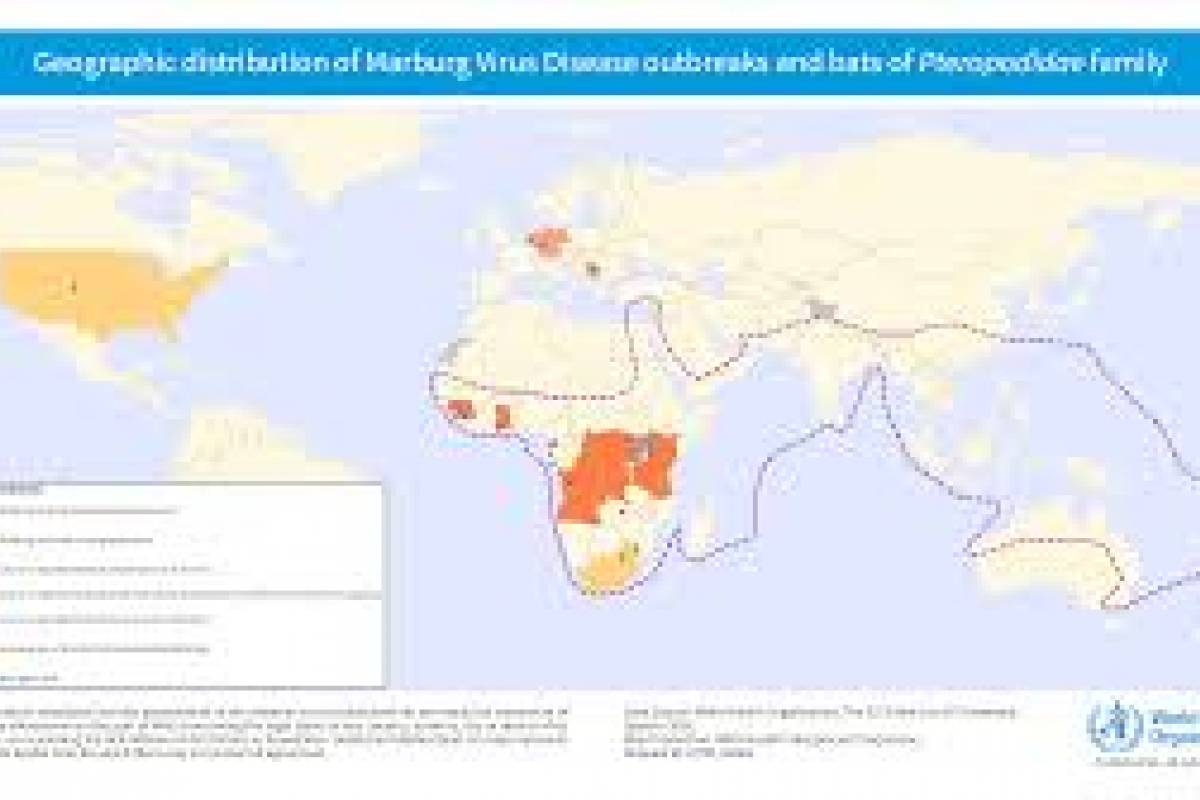
MARV is a member of the Filoviridae family, which also includes Sudan and Zaire ebolaviruses. MARV outbreaks were first recognized in 1967 In Germany and Serbia, and continue in 2024.
MarVax was developed with Dr. Axel Lehrer at the University of Hawaiʽi at Mānoa.
In addition to providing a seven-year term of market exclusivity upon final FDA approval, orphan drug designation also positions Soligenix to be able to leverage a wide range of financial and regulatory benefits, including government grants for conducting clinical trials, waiver of expensive FDA user fees for the potential submission of a Biologics License Application, and certain tax credits.
The U.S. Orphan Drug Act assists companies in developing safe and effective therapies for treating rare diseases and disorders that affect fewer than 200,000 people in the U.S.
Christopher J. Schaber, PhD, President and Chief Executive Officer of Soligenix, commented in a press release on April 15, 2024, "Elements of this subunit vaccine platform... indicate its broad applicability."
"We have also demonstrated the ability to package more than one vaccine antigen in a single vaccine, particularly against MARV and Sudan ebolavirus where there are currently no available vaccines."
"The FDA's decision to grant orphan drug designation to both the MARV and Sudan ebolavirus vaccine candidates signifies an important step for Soligenix as we continue to advance the program and adds significantly to the existing patent estate surrounding this novel technology and the filovirus program."
As of April 2024, several MARV vaccine candidates are conducting early-stage clinical studies.
Our Trust Standards: Medical Advisory Committee