GSK's Meningococcal ABCWY Vaccine Candidate Coming in 2025

The U.S. FDA has set February 14, 2025, as the action date for a GSK MenABCWY combination vaccine candidate that protects against the five most common groups of bacteria causing invasive meningococcal disease.
GSK’s 5-in-1 MenABCWY vaccine candidate combines the antigenic components of its two vaccines with demonstrated efficacy and safety profiles, Bexsero® (MenB-4C) and Menveo (Meningococcal [Groups A, C, Y, and W-135] Oligosaccharide Diphtheria CRM197 Conjugate Vaccine).
Combining the protection offered by these U.S. FDA-approved vaccines with fewer shots will reduce and simplify the number of injections.
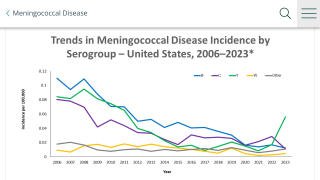
In the U.S., while meningococcal vaccine recommendations for all five serogroups have been in place since 2015, annual immunization rates have remained low overall due in part to a complex vaccination schedule.
As of April 16, 2024, there are various approved meningococcal vaccines and candidates conducting clinical research.
Our Trust Standards: Medical Advisory Committee