Can Increasing Cholera Vaccine Supply Save Lives

Many countries are facing devastating outbreaks of cholera in 2024 due to inadequate supplies of oral cholera vaccines (OCV).
The World Health Organization (WHO) has classified the global resurgence of cholera outbreaks as a grade 3 emergency, its highest alert level.
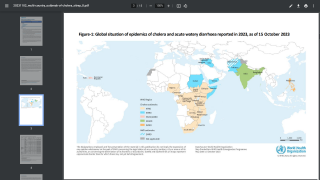
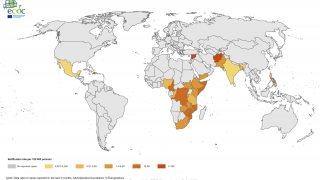
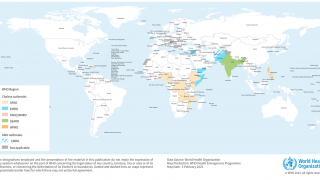
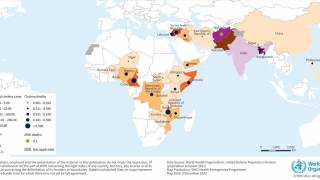
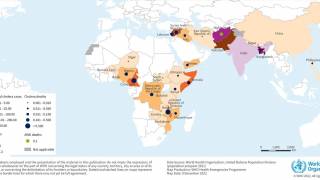
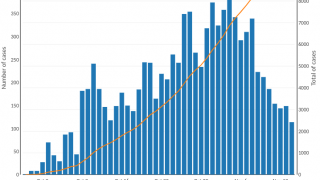
The WHO reported in their multi-country outbreak of cholera, External Situation Report #13, that since the beginning of 2023, and as of March data, there have been a cumulative total of 824,479 cholera cases and approximately 5,900 deaths reported from 31 countries.
Around the same time, in 2023, 62,199 cases and 326 cholera-related deaths were reported from 22 countries.
Over 20% of suspected cholera cases are in young children.
Based on the number of outbreaks and their geographic expansion, alongside the shortage of vaccines and other resources, WHO re-assed the risk at the global level as very high, and the event remains classified as a grade 3 emergency as of April 17, 2024.
"Cholera's true toll often remains obscured by underreporting, masking the full extent of its impact on communities worldwide," stated Duellyn Pandis, DNP, MS, APRN, FNP-C. "Underreporting hampers efforts to allocate resources, implement timely interventions, and prevent the further spread of the disease."
"Cholera vaccines provide a crucial line of defense against the devastating effects of the disease, offering protection to individuals and communities at risk. By stimulating the immune system to recognize and combat the cholera bacterium, vaccines reduce the likelihood of infection and the severity of outbreaks."
"Access to cholera vaccines not only saves lives but also helps prevent the spread of the disease, contributing to healthier populations and stronger public health systems," added Pandis, President & CEO of Passport Health of Tampa Bay.
The WHO recommends cholera vaccination in areas where local transmission of cholera occurs. However, the response in five WHO regions continues to be affected by a critical shortage of OCVs.
Since January 2023, OCV requests have surged, with 79 million doses requested by 14 countries, double the 40 million doses available during this period.
The global stockpile of vaccines was depleted until the beginning of March 2024.
As of April, the stockpile has 2.3 million doses, below the global target of five million.
According to the WHO, there are four OCVs: Vaxchora, Dukoral, ShanChol, and Euvichol-Plus/Euvichol.
On April 17, 2024, EuBiologics and the International Vaccine Institute (IVI) announced that Euvichol-S®, an improved OCV, achieved WHO prequalification. EuBiologics is currently the only OCV supplier to the global stockpile.
Euvichol-S is a new OCV that has improved productivity by about 40% over the existing Euvichol-Plus® by modifying the formulation and manufacturing method of the material.
According to a press release, EuBiologics can now supply three oral cholera vaccines, including Euvichol® (in glass vials) and Euvichol-Plus®.
After the scale-up process, Euvichol-S will be mass-produced in the Republic of Korea.
Dr. Julia Lynch, Director of IVI's Cholera program, stated, "The addition of Euvichol-S to the global health market will contribute to easing the shortage of OCV supply amid a dire global cholera situation."
"IVI will continue efforts to enhance the availability of OCV worldwide and develop new and improved vaccines that are equally safe, effective, and affordable."
The Bill & Melinda Gates Foundation funded the development of Euvichol-S.
The U.S. CDC recommends that adults traveling to areas with active cholera transmission get vaccinated. Over the past two years, the CDC has issued various Travel Health Advisories identifying cholera outbreaks.
Before departing abroad, the CDC suggests discussing vaccination options with a travel vaccine expert in the U.S.
Our Trust Standards: Medical Advisory Committee