Mpox Vaccine and Treatment Access Expansion Includes Pharmacy

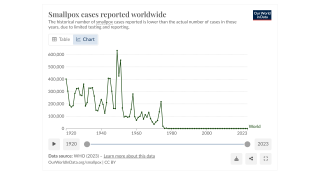
Although mpox is no longer considered a public health emergency, infections are still reported worldwide.
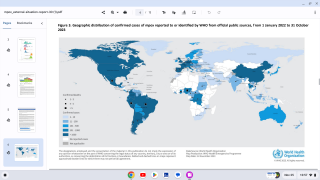
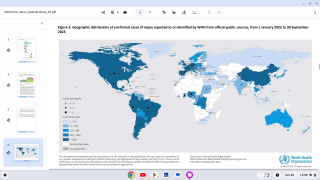
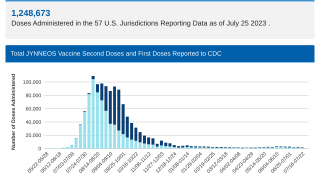
Since the initial outbreak of mpox in May 2022, over 32,000 cases have been reported in the United States, accounting for a third of all global cases.
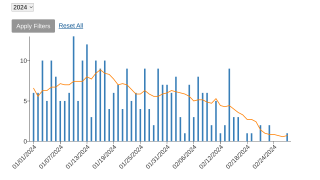
As of April 2024, around 200 monthly cases of mpox are being reported, and the sexually transmitted virus has spread across most of the United States.
However, there is good news regarding expanded access to preventive vaccines and treatments.
Bavarian Nordic A/S announced today that JYNNEOS®, the only FDA-approved mpox vaccine, is now commercially available in the U.S.
Since 2022, in response to the global mpox outbreak, JYNNEOS has been made available through public health channels for individuals at risk of mpox infection. This was enabled by interim guidance from the U.S. Centers for Disease Control and Prevention (CDC), recommending pre- and post-exposure vaccine use for at-risk individuals during the outbreak.
These recommendations were updated in October 2023 by a unanimous vote by the CDC's Advisory Committee on Immunization Practices. JYNNEOS is now recommended for routine use in individuals 18 and older with certain risk factors.
From a mpox treatment perspective, SIGA Technologies, Inc. announced on April 1, 2024, that it entered into an amendment to its international promotion agreement with Meridian Medical Technologies, Inc. regarding oral TPOXX® (Tecovirimat).
Effective June 1, 2024, SIGA will drive international promotion activities for TPOXX while maintaining its contractual relationship with Meridian to maintain continuity for key customer relationships.
In 2018, SIGA signed a contract with the Biomedical Advanced Research and Development Authority for additional procurement and development related to TPOXX's oral and intravenous formulations.
While mpox no longer constitutes a public health emergency, infections are still occurring throughout the U.S. Since the beginning of the outbreak in May 2022, more than 32,000 cases have been reported in the U.S.
According to estimates from the U.S. CDC, two million individuals are currently eligible for mpox vaccination. Recent data shows that both pre- and post-infection vaccination offers measurable benefits.
The CDC's adult immunization schedule for 2024 includes updates for mpox vaccination.
As of April 1, 2024, JYNNEOS is available for at-risk individuals at local pharmacies, physician offices, and public health clinics.
Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, commented in a press release, "From the beginning of the mpox outbreak, almost two years ago, the prompt availability of an approved vaccine combined with a strong public health response has helped to significantly reduce the impact of this debilitating disease, but unfortunately, mpox has not gone away completely."
"Building on the trust and reliability as a supplier of vaccines to the U.S. government for more than a decade, we are proud to extend our commitment to improving the nation's public health by making our mpox vaccine widely available to at-risk individuals through the regular channels."
"We look forward to working with healthcare providers nationwide to increase awareness and availability of the mpox vaccine."
Our Trust Standards: Medical Advisory Committee