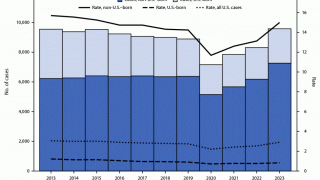
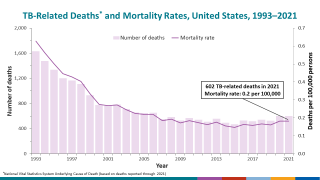
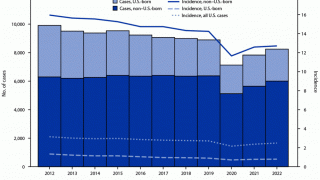
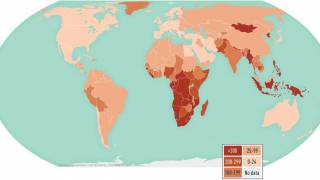
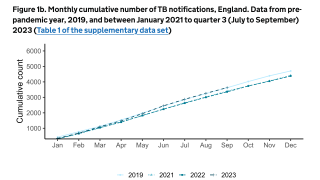
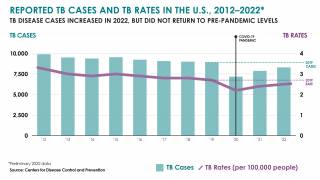
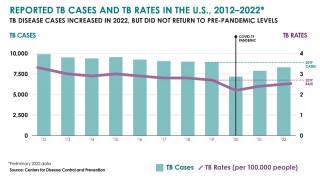
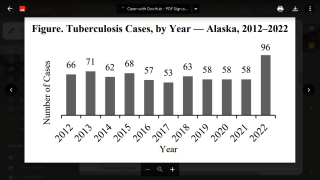
TB Vaccine Candidate Plans Efficacy Study

Biofabri and IAVI recently announced signing an agreement for the end-to-end development of tuberculosis (TB) vaccine candidate MTBVAC. This agreement provides a framework for the future collaboration that the partners first announced in 2021.
After securing sufficient funding, IAVI plans to begin an efficacy clinical trial in 2024.
MTBVAC is a highly promising vaccine candidate that has the potential to be used as an alternative to BCG vaccination in infants and for the prevention of TB disease in adolescents and adults.
"The world urgently needs a new, effective vaccine that can prevent TB disease in adults, adolescents, and infants," said Dr. Mark Feinberg, president, and CEO of IAVI, in a press release on May 17, 2023.
"We are honored to work with Biofabri and our other collaborators to advance MTBVAC."
"In addition, we are actively seeking the support of global health funders and other partners, public and private, to ensure that this promising vaccine candidate has the potential to be part of a solution to ending the TB epidemic."
Should MTBVAC be safe and efficacious, Biofabri will ensure that the TB vaccine is manufactured and supplied in sufficient quantities globally and is accessible at affordable prices in low- and middle-income countries.
Precision Vaccinations post other TB vaccine and outbreak news.
Our Trust Standards: Medical Advisory Committee