Second Generation Oral Mpro Inhibitor for COVID-19 Treatment Proceeds in Phase 3 Study
Sorrento Therapeutics, Inc. today announced the full enrollment in a pivotal Phase 3 study of the oral Mpro inhibitor, Ovydso (STI-1558), in mild or moderate symptomatic adults infected with SARS-CoV-2, or COVID-19.
Sorrento anticipates that top-line data from the study will be available in the third quarter of 2023.
Once the data is finalized, Sorrento plans to open discussions with regulatory authorities worldwide to discuss the path required for each particular authority for full approval of Ovydso.
If the trial meets its endpoints, the company has agreements with the China Health Authority and the National Medical Products Administration (NMPA) for an application review.
“We are pleased to see that Ovydso has enrolled quickly for successful completion of enrollment for the phase 3 pivotal trial in China. We look forward to seeing the final data and to working closely with the NMPA during the review to evaluate this as a potential stand-alone treatment for COVID-19 patients as rapidly as possible,” stated Henry Ji, Ph.D., Chairman and CEO of Sorrento, in a press release on June 26, 2023.
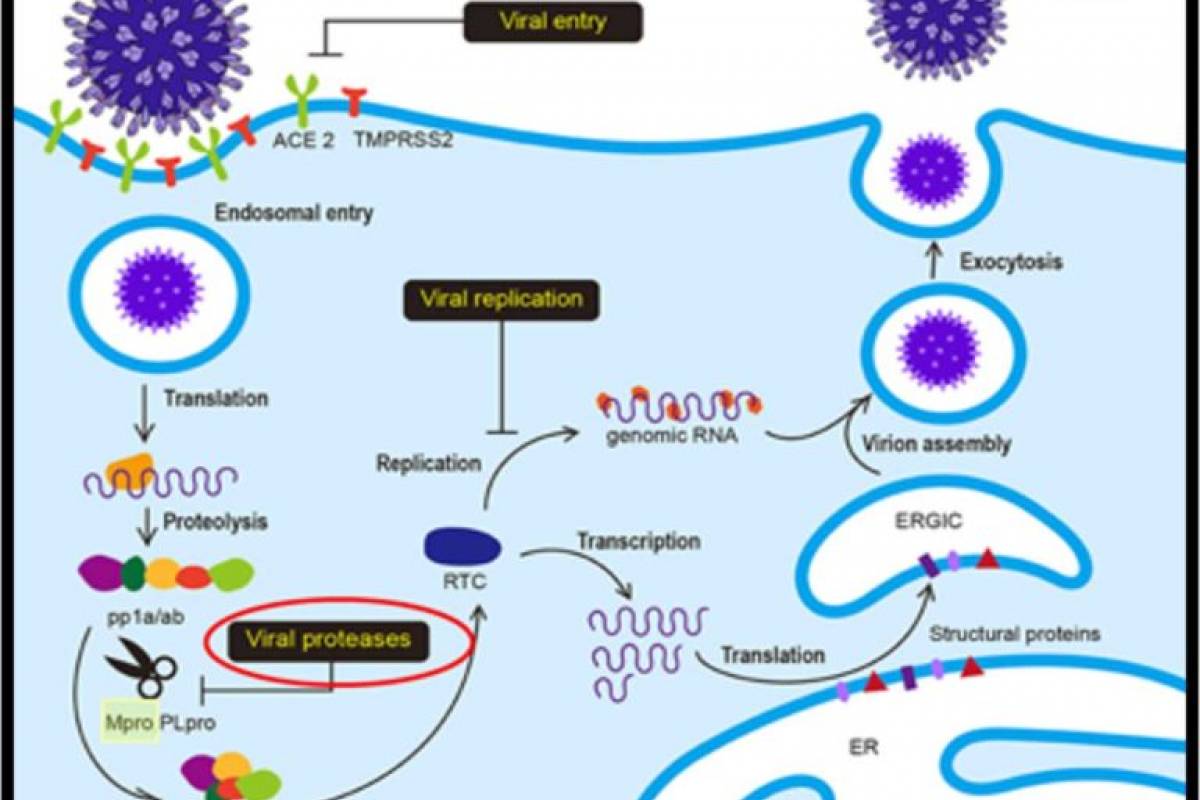
STI-1558 is a prodrug, and its active form AC1115 binds to Cys-145 of the catalytic domain of Mpro, which is 100% conserved in all SARS-CoV-2 variants and achieves a broad-spectrum anti-SARS-CoV-2 activity.
STI-1558 is also a Cathepsin L inhibitor, which may block effective viral entry into host cells without accelerating viral mutations.
Sorrento is a clinical and commercial stage biopharmaceutical company based in California.
Our Trust Standards: Medical Advisory Committee























