20 Million Novel Polio Vaccines Heading to Indonesia

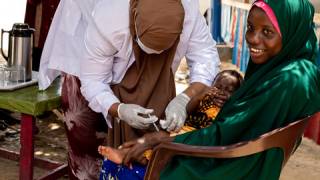
In response to an active polio outbreak, the Indonesian government recently requested the WHO-Director General’s approval to release novel oral polio vaccine type 2 (nOPV2).
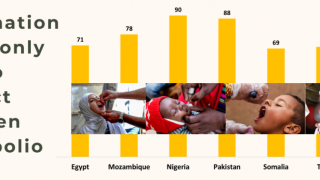
As of January 11, 2024, the WHO issued a Disease Outbreak News alert (DON500) confirming the approval of more than 20 million doses of nOPV2 to conduct two rounds of supplementary immunization activities scheduled on January 15, 2024, and February 19, 2024.
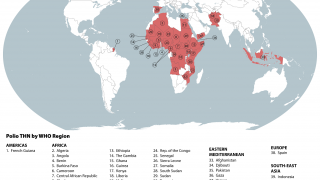
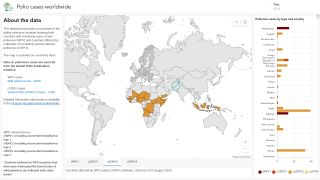
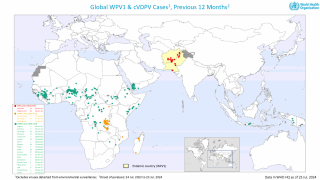
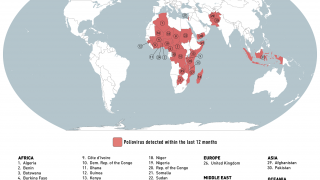
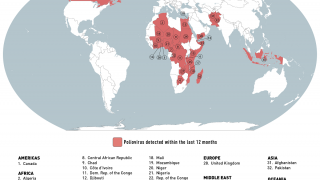
Previously, Indonesia reported four cases of circulating vaccine-derived poliovirus type 2 (cVDPV2) from October 2022 to February 2023.
In late December 2023, the Indonesian Ministry of Health notified WHO of two new confirmed cases of cVDPV2.
Genetic sequencing of isolates at the BioFarma National Polio Laboratory indicates cVDPV2 with 36 nucleotide changes, genetically linked to a West Java province case reported to WHO in March 2023.
Vaccine-derived poliovirus is a well-documented strain mutated from the strain initially in oral polio vaccines.
In sporadic instances, the vaccine-derived virus can genetically change into a form that can cause paralysis, as does the wild poliovirus – this is what is known as a vaccine-derived poliovirus (VDPV).
The detection of VDPV in at least two different sources and at least two months apart that are genetically linked and showing evidence of transmission in the community is classified as cVDPV.
According to the WHO, the overall risk is assessed as high at the national level. At the regional level, the overall risk is assessed to be moderate.
Furthermore, the WHO advises against implementing any travel or trade restrictions on Indonesia based on the current information available on this event.
As of January 12, 2024, about 1 billion doses of the nOPV2 vaccines have been recently distributed.
Our Trust Standards: Medical Advisory Committee