Enhanced Tuberculosis Vaccine Candidate Launches Phase 3 Study In India

A new tuberculosis (TB) vaccine candidate, MTBVAC, has started clinical trials in India. This vaccine is the first live attenuated vaccine of Mycobacterium tuberculosis isolated from a human.
Bharat Biotech is conducting the clinical trials in collaboration with Biofabri, S.L.U, the vaccine's owner. The phase 3 clinical trials of the vaccine were announced on March 23, 2024, and plan to start in 2025.
The MTBVAC vaccine has passed several milestones before entering clinical trials in India.
This is a significant development since India has a high incidence of TB cases.
Esteban Rodriguez, CEO of Biofabri, commented in a press release, "It is a giant step to test in adults and adolescents in the country where 28% of the world's TB cases accumulate."
"It should be remembered that the only vaccine in use today, Bacillus Calmette and Guérin (BCG), is an attenuated variant of the bovine TB pathogen. It has a very limited effect on pulmonary tuberculosis, which is responsible for transmitting the (respiratory) disease."
Unlike BCG, which lacks over 100 genes compared to the human pathogen, MTBVAC contains the complete set of antigenic targets of the original pathogen.
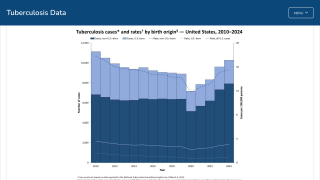
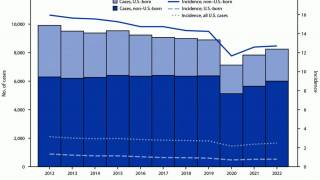
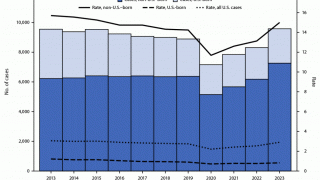
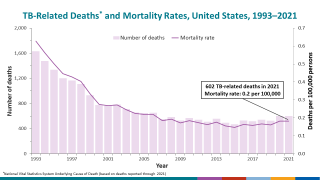
According to the World Health Organization (WHO), the number of people who fell ill with TB in 2021 was 4.5% higher than in 2020, with 10.6 million people diagnosed.
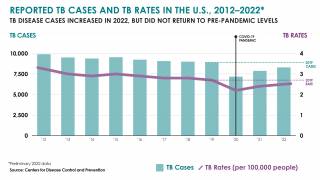
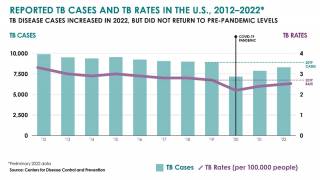
In 2023, India reported over 2.2 million TB cases.
Biofabri is part of the Zendal Group, a Spanish pharmaceutical company specializing in human and animal health.
Our Trust Standards: Medical Advisory Committee