Oral Cholera Vaccines Needed in 2024 to Curtail Outbreaks
The World Health Organization (WHO) today announced an updated External Situation Report #17 on a multi-country outbreak of cholera.
Published on August 15, 2024, the global cholera response continues to be affected by a critical shortage of Oral Cholera Vaccines (OCV) as demand continues to outpace supply.
Since January 2023, 18 countries have requested 105 million doses, nearly double the 55 million doses produced in this period.
Furthermore, in January 2023, the WHO classified the global resurgence of cholera as a grade 3 emergency, the highest internal level for emergencies in WHO.
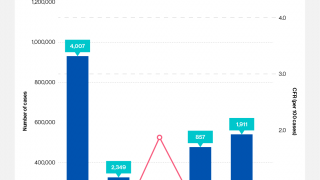
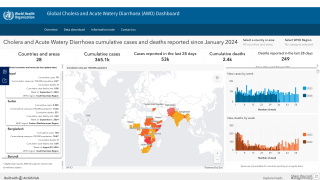
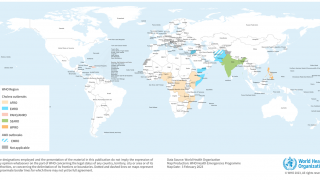
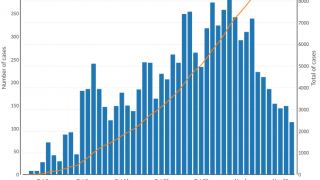
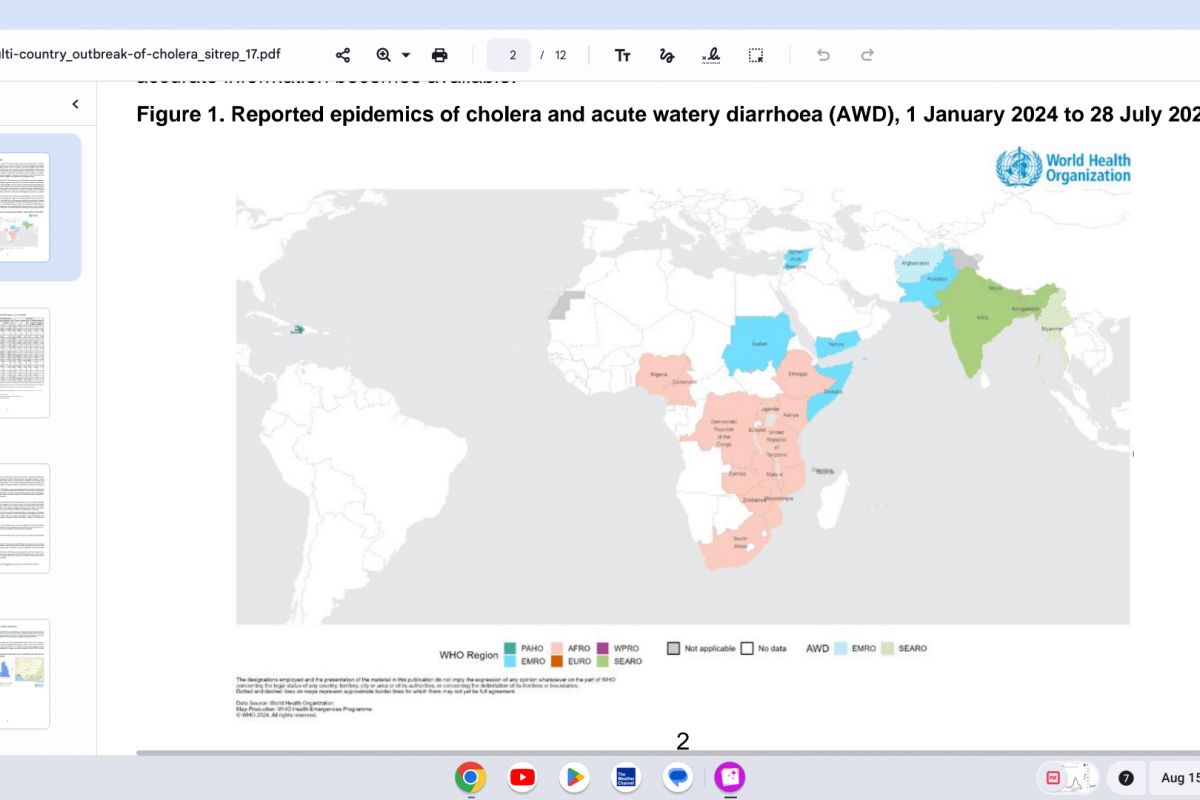
As of the end of July 2024, a cumulative total of 307,433 cholera cases and 2,326 deaths were reported from 26 countries across five WHO regions.
Based on the number of outbreaks and their geographic expansion, alongside the shortage of vaccines and other resources, WHO continues to assess the risk at the global level as very high, and the event remains classified as a grade 3 emergency.
As of August 2024, the WHO and other governments have authorized four OVCs.
For example, Valneva SE's DUKORAL® is an oral, inactivated vaccine for diarrhea prevention authorized in various countries.
DUKORAL contains four different inactivated strains of V. cholerae serotype O1 and part of a toxin from one of these strains as active substances. Valneva recently announced that DUKORAL® sales totaled €14.9 million for the first half of 2024.
In the United States, cholera vaccines are in limited supply in 2024, but available at certain travel vaccine pharmacies.
Our Trust Standards: Medical Advisory Committee