Mass Mpox Vaccination Not Recommended
The World Health Organization (WHO) recently announced that mass mpox vaccination is not recommended against this sexually transmitted disease.
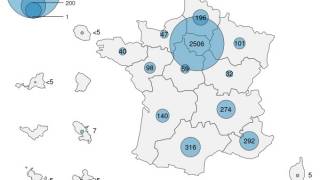
On August 17, 2024, the WHO confirmed international travelers who may be at risk based on an individual assessment with their healthcare provider may wish to consider vaccination before visiting countries reporting mpox outbreaks, such as in Africa.
Recently, the WHO Director-General announced that he had triggered the process for Emergency Use Listing of mpox vaccines. The WHO currently recommends two new vaccines against mpox disease and continues listing an older smallpox vaccine as an option.
The United States Food and Drug Administration (FDA) Approved Bavarian Nordic's JYNNEOS® (MVA-BN, IMVANEX®, IMVAMUNE®) Smallpox and Mpox Vaccine on September 24, 2019. The U.S. began offering the JYNNEOS vaccine to healthcare staff in Boston on May 24, 2022, in response to the Clade 2 mpox global outbreak.
JYNNEOS remains available in the U.S. at certain clinics and pharmacies.
Additionally, Japan's K.M. Biologics' LC16 "KMB" freeze-dried smallpox vaccine has been approved by the WHO, Japan's Ministry of Health, Labor, and Welfare, and other countries.
The older ACAM2000® live vaccinia virus vaccine is authorized to prevent mpox and smallpox infections in various countries. However, the safety profile of ACAM2000 vaccination includes risks for myocarditis and pericarditis.
The WHO writes, 'Results from vaccine effectiveness studies indicate that a good level of protection is provided against mpox (Clade 2) following vaccination. Further studies on the use of vaccines for mpox (Clade 1) will provide additional information on the effectiveness of these vaccines in different settings.'
Our Trust Standards: Medical Advisory Committee