Vaccination Prevents Chikungunya Disease in Adolescents
The peer-reviewed journal The Lancet Infectious Diseases recently published interim results of a double-blind, randomized, placebo-controlled phase 3 trial in adolescents of the U.S. FDA-approved, single-dose IXCHIQ® (VLA1553) chikungunya vaccine.
In an article published on September 4, 2024, these researchers concluded that VLA1553 was generally safe and induced seroprotective titers in almost all vaccinated adolescents, with favorable safety data in seropositive adolescents at baseline.
VLA1553 induced seroprotective chikungunya virus neutralizing antibody levels in 247 of 250 (98.8%, 95% CI 96·5–99·8) participants 28 days after vaccination.
This data supports using VLA1553 to prevent diseases caused by the chikungunya virus among adolescents and in endemic areas in the Region of the Americas.
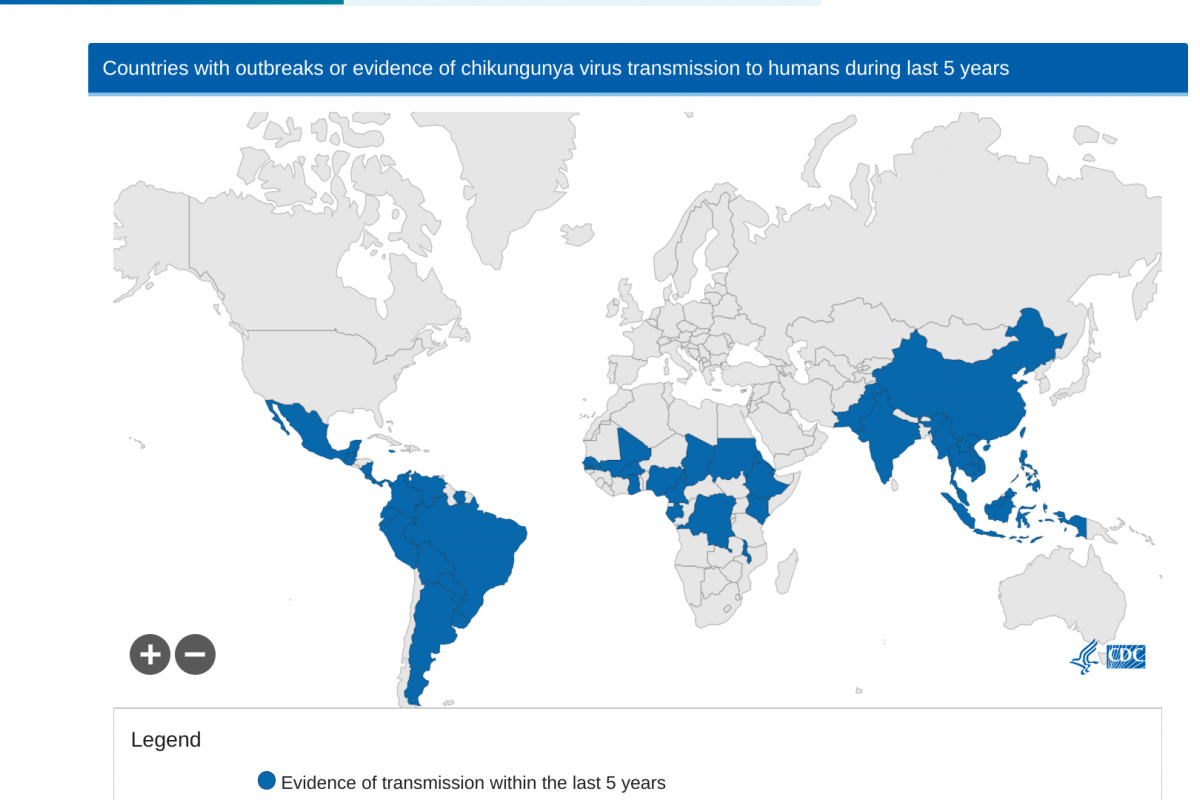
As of September 22, 2024, the Pan American Health Organization reported 390,669 CHIKV cases. Specifically, Brazil has confirmed 170 related deaths this year.
If you are traveling to an area at risk for chikungunya, the U.S. CDC suggests discussing vaccination options with your healthcare provider at least one month before departing abroad.
Our Trust Standards: Medical Advisory Committee