1,700 Marburg Vaccines Deployed to Africa
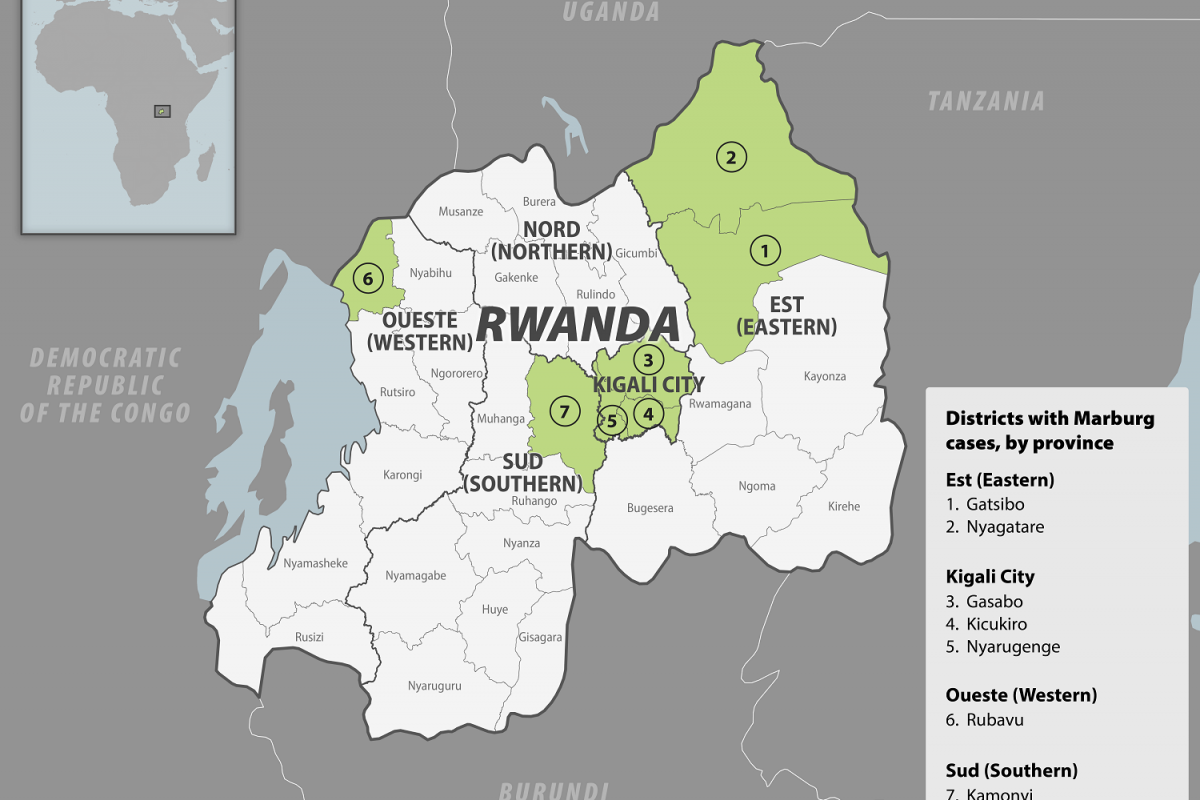
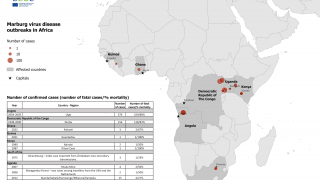
In early October 2024, the Republic of Rwanda began vaccinating frontline health workers in a Phase 2 rapid response open-label clinical trial to combat the reaction to the ongoing Marburg virus disease (MVD) outbreak, which has already claimed 14 lives.
Sabin Vaccine Institute’s single-dose Marburg vaccine candidate was selected to be administered in accordance with the clinical protocol reviewed and approved by Rwandan ethics and regulatory authorities. However, this is not a U.S. FDA-approved vaccine.
As of October 12, 2024, Sabin announced it had delivered approximately 1,700 investigational vaccine doses to Rwanda.
“In an outbreak, every moment counts, and our seamless collaboration with the Rwandan government was key to accelerating the process. On our side, we moved quickly by leveraging our experience with other outbreaks and having vaccine doses and supporting documents ready, thanks to a strong partnership with ReiThera,” says Sabin's CEO Amy Finan in a press release.
Sabin has extensive expertise in advancing vaccines for filoviruses, with two programs currently in Phase 2 clinical trials—one for Marburg and the other for Sudan ebolavirus.
The U.S. government has obligated $235 million to Sabin to advance vaccine research and development against Sudan ebolavirus and MVD.
As of October 13, 2024, other MVD vaccine candidates are conducting clinical research.
Previously, the U.S. CDC announced that people should reconsider nonessential travel to the Republic of Rwanda and that those who arrive in the U.S. may be screened for the virus at certain airports.
Our Trust Standards: Medical Advisory Committee