Mpox Vaccine Candidate Found Less Virulent
Tonix Pharmaceuticals Holding Corp. today announced the publication of a paper entitled, “Recombinant Chimeric Horsepox Virus (TNX-801) is Attenuated Relative to Vaccinia Virus Strains in Both In Vitro and In Vivo Models.”
Data published in the peer-reviewed journal mSphere demonstrate that TNX‐801 is less virulent than 20th-century vaccinia vaccines in immune-compromised mice.
The publication describes data in which TNX-801 was compared with older vaccinia vaccine strains used to eradicate smallpox for tolerability in both in vitro and in vivo models.
Together, TNX-801 was shown to be more than 10—to 1,000-fold more attenuated (or less virulent) than the older vaccinia smallpox vaccines.
Combined with the ability of TNX-801 to protect animals against lethal challenge with Clade Ia mpox virus, the Company believes that the new tolerability data support the idea that TNX-801 is a candidate vaccine to control the ongoing mpox Clade Ib and mpox Clade IIb outbreaks.
Previously, single-dose vaccination with TNX-801 was shown to protect animals from a lethal challenge with Clade Ia mpox.
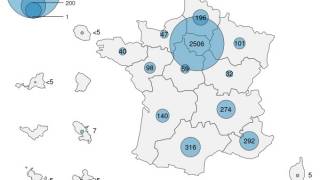
The new Clade Ib mpox is spreading among children in Africa and, so far, has been carried by adult travelers from Africa into non-African countries.
The global mpox outbreak from Clade IIb, which commenced in May 2022, continues in various countries, including the United States.
“Addressing the new Clade Ib mpox outbreak and the ongoing spread of Clade IIb mpox may require a single dose mpox vaccine that provides durable protection,” said Seth Lederman, M.D., Chief Executive Officer of Tonix Pharmaceuticals, in a press release on November 13, 2024.
The current standard of prevention focuses on Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) two-dose vaccine, which is commercially available in the United States.
Our Trust Standards: Medical Advisory Committee