A Better But Not Perfect Polio Vaccine

The World Health Organization (WHO) Director-General recently convened the thirty-fifth meeting of the Emergency Committee under the International Health Regulations on the international spread of poliovirus.
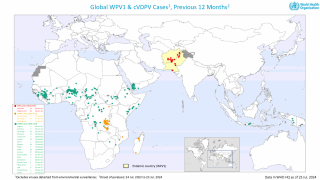
On May 3, 2023, the Committee reviewed data on wild poliovirus (WPV1) and circulating vaccine-derived polioviruses (cVDPV) in the context of the global target of eradication of WPV and cessation of outbreaks of cVDPV2 by the end of 2023.
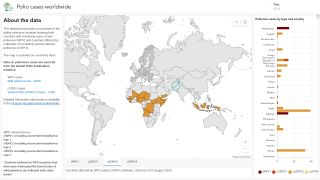
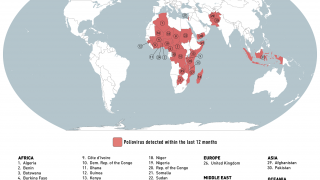
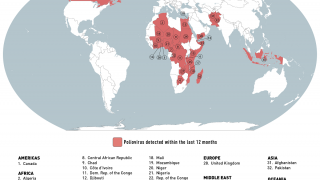
Globally there remain only three genetic clusters of WPV1, a significant reduction in the genetic diversity of WPV1 which indicates that chains of transmission have been reduced to two in the remaining endemic countries, Pakistan and Afghanistan, and one in Africa.
This WHO technical update included the Democratic Republic of the Congo (DR Congo).
The WHO committee noted that in the African Region, which now uses novel OPV2 (nOPV2), two new cVDPV2 detected in DR Congo have emerged from nOPV2's use.
Approximately 600 million nOPV2 doses have been administered in more than 28 countries worldwide as of May 2023.
However, nOPV2 retains its enhanced genetic stability compared to Sabin oral polio vaccine (OPV2), with most isolates analyzed through whole genome sequencing indicating no or minimal changes in the genetic structure of nOPV2.
Only 2% of all isolates reported so far have shown evidence of losing essential genetic modifications that reduce neurovirulence due to recombination, and these have only been detected in Africa versus the expected 75% for Sabin OPV2.
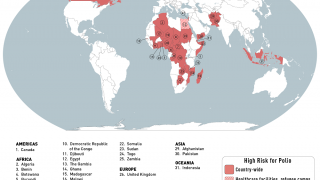
Although encouraged by the reported progress, the WHO Committee unanimously agreed that the risk of the international spread of poliovirus remains a Public Health Emergency of International Concern and recommended the extension of Temporary Recommendations for a further three months in 2023.
In the U.S., the Centers for Disease Control and Prevention (CDC) reissued its Alert - Level 2, Practice Enhanced Precautions regarding polio outbreaks.
According to CDC, 70% of people infected with polio experience no symptoms.
A smaller proportion of people will develop more severe symptoms that affect the brain and spinal cord, including Paresthesia, Meningitis, and Paralysis.
As of March 2023, the CDC says adults who previously completed the whole routine polio vaccine series receive a single, lifetime booster dose of polio vaccine before traveling to any listed destination.
While the nOPV2 vaccine is not offered in the U.S., the inactivated polio vaccine (IPV) has been given since 2000.
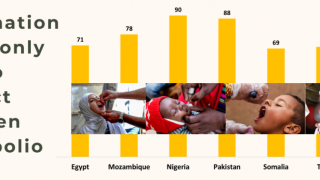
Vaccine effectiveness estimates (Feb. 2023) against paralytic polio range from 36%–89% for one IPV dose, and vaccination appears to reduce the mean quantity of shed poliovirus by 63%–91%.
As of May 27, 2023, polio vaccinations are offered at health clinics and community pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee