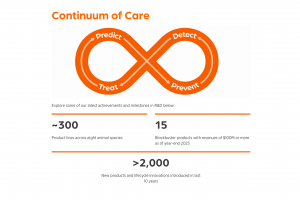
Avian Influenza Vaccines
Avian Influenza Vaccines
The World Health Organization (WHO) reported in February 2025 that 1,074 human cases of influenza A have been reported globally since 2003. These cases include H5, H5N1, H5N6, and H5N8 virus strains. As of March 2025, U.S. FDA-approved avian influenza vaccines are not commercially available in the U.S. Furthermore, the FDA and the United Kingdom and European agencies say annual flu shots are unlikely to protect people during avian/pandemic influenza (bird flu) pandemics. On December 11, 2024, the U.S. administration informed the media there are no active plans to authorize the distribution of avian influenza (bird flu) vaccines. On June 27, 2024, the U.S. Centers for Disease Control and Prevention (CDC) confirmed its avian vaccination program was inactive.
The U.S. National Influenza Vaccine Modernization Strategy and the American Pandemic Preparedness Plan outlined the United States' priorities for avian and pandemic influenza vaccine development and deployment. As of 2025, the U.S. Influenza & Emerging Infectious Diseases (EID) Pandemic Vaccines and Adjuvants Program has advanced vaccination strategies to mitigate future pandemics. It also manages the U.S. National Pre-Pandemic Influenza Vaccine Stockpile (NPIVS) and implements its strategic use in the event of a public health emergency. The NPIVS utilizes the HHS Influenza Risk Assessment Tool (IRAT), updated in July 2023 and run by the CDC, to identify and evaluate potential pandemic influenza virus threats.
On April 21, 2024, the APHIS National Veterinary Services Laboratories made available 239 genetic sequences from the H5N1 clade 2.3.4.4b influenza virus recently found in samples associated with the ongoing highly pathogenic avian influenza (HPAI) outbreak in the U.S. H5N1 is divided into several clades, each with its geographic and clinical significance. According to the CDC, about 20 million H5N1 and 12 million H7N9 vaccines were available in the National Strategic Stockpile as of June 2023. The CDC also confirmed in October 2023 that an H5 candidate vaccine virus (CVV) similar to the hemagglutinin (HA) protein of H5N1 clade 2.3.4.4b A(H5) Candidate Vaccine Virus Development (pg. 37). The CDC says this H5 CVV could be used to produce a highly pathogenic avian influenza (HPAI) A(H5N1) virus vaccine for people.
Avian Influenza Vaccine Effectiveness
A Brief Communication published by the journal Nature Medicine on July 16, 2024, included the stockpiled US-licensed adjuvanted H5N1 vaccines. These vaccines generate cross-neutralizing antibodies against circulating HPAI H5N1 clade 2.3.4.4b in humans and may be helpful as bridging vaccines until updated H5N1 vaccines become available.
U.S. FDA-Approved Avian Influenza Vaccines
On September 15, 2009, four influenza vaccine manufacturers received approval from the FDA to use the influenza A (H1N1) 2009 monovalent influenza vaccine to prevent outbreaks caused by the 2009 pandemic influenza A (H1N1) virus. BARDA is partnering with GSK and CSL Seqirus to manufacture investigational lots of H5N8 vaccines and clinically assess adjuvants' safety, immunogenicity, and dose-sparing ability combined with the manufactured vaccines.
The U.S. FDA authorized CSL Seqirus Inc. Audenz™ (Influenza A(H5N1) Monovalent Vaccine, Adjuvanted) cell-based vaccine on January 31, 2020. CSL Seqirus has produced zoonotic vaccines to address the H5N1 virus threat using egg-based technology at its Liverpool, UK facility (virus grown in eggs) and cell-based technology at its Holly Springs, NC facility (virus grown in cells).
On November 14, 2013, the FDA licensed the I.D. Biomedical Corporation Influenza A (H5N1) Virus Monovalent Vaccine, Adjuvanted (STN#: 125419), to prevent H5N1 influenza disease. The vaccine was not commercially available in 2024, but the U.S. federal government purchased it for the National Stockpile for as-needed distribution.
Sanofi Pasteur's Influenza Virus Vaccine, H5N1, was FDA-approved in 2007.
Through six awards, CSL Seqirus has been working with the U.S. BARDA to support U.S. pandemic preparedness objectives. On October 4, 2024, CSL Seqirus received a new BARDA award to complete the fill-and-finish process for additional pre-pandvaccinesccine. This $34 million investment further supports the U.S. government's outbreak and preparedness response. It is the sixth avian influenza pandemic preparedness award made to CSL Seqirus by BARDA. On September 25, 2024, CSL Seqirus announced an award of $121.4 million. As part of the NPIVS, it would expand its reserves of MF59® adjuvant to the equivalent of 40 million doses and expand its Vendor-Managed Inventory program for its proprietary MF59® adjuvant.
On May 30, 2024, CSL Seqirus announced that BARDA selected it to complete the fill-and-finish process of the pre-pandemic avian influenza vaccine for the U.S. government as part of the NPIVS program. The company stated it intends to deliver approximately 4.8 million doses of pre-pandemic vaccine that are well-matched to the H5 component of the currently circulating H5N1 strain.
CSL Seqirus announced on August 28, 2023, that BARDA selected the company to deliver one bulk lot of H5N8 A/Astrakhan antigen to support the U.S. government's pandemic response readiness under contract number 75A50122D00004 ($46.3 million). Seqirus received an award from BARDA to produce an H5N8 A/Astrakhan virus vaccine seed and the subsequent October 2022 announcement of the selection of CSL Seqirus to deliver an H5N8 A/Astrakhan virus vaccine candidate for assessment in a Phase 2 clinical study. Under the $30.1 million agreement, CSL Seqirus has established and maintains the required pandemic readiness to deliver 150 million doses of cell-based pandemic influenza vaccine within six months of an influenza pandemic declaration in the U.S. In 2022, Sequirus's Holly Springs facility successfully achieved BARDA's criteria to establish domestic manufacturing capability for innovative cell-based seasonal and pandemic influenza vaccines.
On October 5, 2022, CSL announced results from the preclinical studies of the company's self-amplifying messenger RNA (sa-mRNA) influenza vaccine candidates. The data, published in Molecular Therapy – Methods and Clinical Development, indicate that the sa-mRNA influenza vaccine candidates produced a potent, cross-reactive immune response against pandemic and seasonal influenza strains A(H5N1) and A(H1N1) (Swine flu). Roberta Duncan, CSL's mRNA Program Lead, commented in an article in March 2024, "The innovation of sa-mRNA brings the opportunity to provide greater public health protection against respiratory viruses and in other disease states."
Canada Avian Influenza Vaccine
GSK—I.D. Biomedical Corporation of Quebec's Arepanrix H5N1 A/American wigeon clade 2.3.4.4b vaccine is approved by the U.S. FDA. It uses established technology for seasonal and pandemic flu vaccines approved in Canada. On February 19, 2025, the Public Health Agency of Canada released the National Advisory Committee on Immunization's Preliminary guidance on human vaccination against avian influenza in a non-pandemic context as of December 2024.
China Pandemic Influenza Vaccine
SINOVAC's whole viron inactivated H5N1 pandemic influenza vaccine, Panflu®, is supplied to the Chinese government's stockpiling program.
European Avian Influenza Vaccines
According to the European Medicines Agency (EMA), one vaccine (Aflunov) is authorized for use in the European Union (EU) during avian influenza outbreaks without a declared pandemic. Aflunov contains a flu strain called A/turkey/Turkey/1/2005 (H5N1)-like strain (NIBRG-23) (clade 2.2.1). Additionally, the EMA says four pandemic-preparedness influenza vaccines authorized in the EU can be rapidly modified to protect people in a pandemic situation. The EMA's human medicines committee recommended Celldemic at its February 2024 meeting (zoonotic influenza vaccine [H5N1] [surface antigen, inactivated, adjuvanted, prepared in cell cultures]). This vaccine is intended for immunization during outbreaks of influenza coming from animals, including when public health authorities anticipate a possible pandemic. Incellipan (pandemic influenza vaccine (H5N1) (surface antigen, inactivated, adjuvanted, prepared in cell cultures)) is a pandemic preparedness vaccine intended for use only if a flu pandemic has been officially declared.
On June 11, 2024, CSL Seqirus announced it would provide 665,000 pre-pandemic (zoonotic) vaccines to the EC's fifteen EU and EEA Member States. Seqirus UK Ltd has an EU-wide modified marketing authorization for this avian influenza vaccine for use in adults. On June 5, 2024, Andrew Joseph wrote that Finland may become the first country to offer 'bird flu' vaccinations to people this year. The Zoonotic Influenza Vaccine Seqirus is used in Finland.
The European Commission (EC) signed a framework contract on July 28, 2022, for the joint procurement of GSK's Adjupanrix, a pandemic influenza vaccine. As a result, EC Member States can purchase up to 85 million vaccine doses during an influenza pandemic.
Japan Avian Influenza Vaccines
Japan's Health Ministry announced in May 2023 that it is changing its avian influenza vaccine stockpile from H7N9 to the H5N1 virus. On May 25, 2023, local media reported that Japan intends to stockpile 10 million vaccines for people. In 2018, Japan began culling infected chickens. In 2008, Japan started testing bird flu vaccine versions. In 2003, after 79 years, a HAPI virus was detected in the dead chickens.
United Kingdom Influenza Pandemic Vaccines
The UK. Health Security Agency announced on September 26, 2023, an advance purchase agreement with CSL Seqirus to produce over 100 million influenza pandemic vaccines if or when they are needed. On December 3, 2024, the UK Government purchased over five million doses of the human H5 influenza vaccine manufactured by CSL Seqirus UK Limited, a UK-based healthcare company.
Avian Influenza Vaccine Adjuvant
According to Vanderbilt University Medical Center research published in The Journal of Infectious Diseases (January 2024), avian (bird) influenza vaccines created a more robust immune response in a phase 1 study paired with the GSK-manufactured AS03 adjuvant.
mRNA Pandemic Influenza Vaccine Candidates
Moderna initiated a Phase 1/2 study in July 2023 to generate safety and immunogenicity data for the investigational pandemic influenza vaccine (mRNA-1018) in healthy adults 18 years and older. The study includes vaccine candidates against H5 and H7 avian influenza viruses. Results from the study are expected in 2025 and will inform Phase 3 development plans. On January 17, 2025, the U.S. government awarded Moderna $590 million to continue vaccine development.
Argentinian manufacturer Sinergium Biotech is leveraging the WHO and the Medicines PatPool's mRNA Technology Transfer Programme. Sinergium has developed candidate H5N1 vaccines and aims to esproof of concept-concept in preclinical models. Once the preclinical data package is concluded, the technology, materials, and expertise will be shared with other manufacturing partners.
In April 2024, the U.S. FDA granted CurVac N.V. Fast Track designation for a monovalent influenza A (H5N1) pre-pandemic mRNA vaccine candidate encoding an H5-antigen. The candidate is being developed in collaboration with GSK. The start of Phase 1 part of a combined Phase 1/2 study was announced on April 24, 2024.
Arcturus Therapeutics HoldInc. 's Inc. ARCT-2304 is a sa-mRNA vaccine candidate formulated within a lipid nanoparticle (LNP). The sa-mRNA vaccine candidate is designed to make many copies of mRNA within the host cell after intramuscular injection to achieve enhanced expression of haemagglutinin (HA) and neuraminidase (NA) antigens, thereby enabling lower doses than conventional mRNA vaccines. The FDA issued a "Study Can Proceed" notification on November 11, 2024.
Vir Biotechnology Pandemic Monoclonal Antibody
On October 4, 2022, the BARDA awarded California-based Vir Biotechnology, Inc. a multi-year contract with a potential value of up to $1 billion to advance the development of a complete portfolio of innovative solutions (vaccines) to address influenza and other infectious disease threats. BARDA will initially invest approximately $55 million for the ongoing and rapid development of VIR-2482, an investigational prophylactic monoclonal antibody (mAb) designed to protect against seasonal and pandemic influenza. On July 20, 2023, Vir announced that the Phase 2 PENINSULA trial evaluating VIR-2482 for preventing symptomatic influenza A illness did not meet primary or secondary efficacy endpoints.
Avian Influenza Vaccines for Birds
Boehringer Ingelheim VAXXITEK® HVT+IBD+H5 is a new trivalent vaccine for poultry that offers protection against Marek's disease, Infectious Bursal Disease, and H5 avian influenza.
The United States Department of Agriculture's Agricultural Research Service (APHIS) researchers confirmed on April 14, 2023, that they are currently testing several vaccine candidates for use with birds. On May 16, 2023, APHIS approved the emergency use of an HPAI vaccine to prevent additional deaths of California Condors. The authorized vaccine is a killed, inactivated product conditionally licensed by APHIS' Center for Veterinary Biologics in 2016.
The DIVA strategy uses an inactivated oil emulsion vaccine containing the same haemagglutinin (H) subtype as the challenge virus but a different neuraminidase (N). The possibility of using the heterologous N subtype to differentiate between vaccinated and naturally infected birds was investigated by developing an "ad hoc" serological test to detect specific anti-N1 antibodies.
The World Organisation for Animal Health (WOAH) announced on June 7 that 2023 it issued a comprehensive report - Animal Health Forum on Avian Influenza – Policy to Action: The Case of Avian Influenza – Reflections for Change. It was recognized that a successful vaccination strategy must rely on authorized (bird flu) vaccines. "Vaccinating is not the end; it is just the beginning. Vaccination application needs to be managed along the supply chain, including a surveillance program to detect active infection in vaccinated animals," said Dr. David Swayne, Disease Expert and Forum rapporteur.