Rabies Vaccine Producer Ordered To Discontinue Production in China

According to the Chinese State Drug Administration on July 15, 2018, rabies vaccine manufacturer Changchun Changsheng Biotechnology Co., Ltd. reported it is under investigation.
The Administration found that the company's freeze-dried human rabies vaccine production had record violations such as “Good Manufacturing Practices”.
The State Drug Administration has requested the Jilin Provincial Food and Drug Administration to take back the "Drug GMP Certificate" (Certificate No.: JL20180024) of the enterprise, ordering the stop of rabies vaccine production and take the initiative Control measures to ensure the safety of the public medication.
No vaccine products involved in the inspection were on sale, and all the vaccines involved have been removed, said the Administration.
According to Changchun Changsheng, its vaccine was registered for use in India, Cambodia, and Nigeria by last year. But it was unclear whether any had been exported.
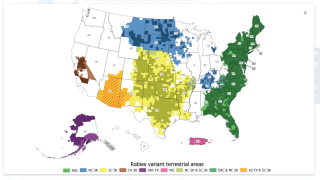
Around the world, rabies in dogs is still a serious health problem. At risk, countries include most of Europe, Japan, Canada, Australia, and India.
Every year in India, 20,000 people are estimated to die from rabies. Nearly all of the deaths occur after victims are bitten by rabid dogs.
Tao Lina, a vaccination specialist at the Shanghai Centre for Disease Control and Prevention, told SCMP “The incident is very severe in nature because it has dealt another blow to the public’s confidence in domestically produced vaccines.”
Changsheng Life Sciences, Changchun Changsheng’s Shenzhen-listed parent company, said in a statement on July 16, 2018, that the company had voluntarily recalled and sealed all the rabies vaccines that had been sent to disease control centers and hospitals.
In the USA, the greatest source of rabies in humans in the USA is from wild carnivores and bats, reports the National Center for Emerging and Zoonotic Infectious Diseases.
The rabies virus attacks the central nervous system, eventually causing disease in the brain and death, says the Centers for Disease Control and Prevention (CDC).
Rabies virus belongs to the order Mononegavirales, viruses with nonsegmented, negative-stranded RNA genomes.
For people who have never been vaccinated against rabies previously, post-exposure anti-rabies vaccination should always include administration of both passive antibody and vaccine.
If people are treated within two or three days after being bitten, the human rabies vaccine is highly effective, and victims usually survive.
The estimated public health expenditures on rabies disease diagnostics, prevention, and control in the US is $245 to $510 million annually.
Although the cost varies, a course of rabies immune globulin and four doses of vaccine given over a two-week period typically exceeds $3,000, reports the CDC.
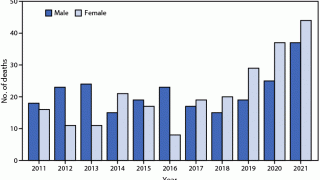
Over the last 100 years, rabies in the United States has changed dramatically. The number of rabies-related human deaths in the United States has declined to one or two per year.
This is because modern day prophylaxis has proven nearly 100% successful, reports the CDC.
The actual number of PEP treatments given in the United States each year is unknown. However, it is estimated by the CDC to be about 40,000 cases.
There are two pre-exposure rabies vaccines approved in the USA:
- Human Diploid Cell Vaccine (HDCV): IMOVAX® RABIES
- Purified Chick Embryo Cell Vaccine (PCEC): RabAvert
A person who is exposed and has never been vaccinated against rabies should get four doses of rabies vaccine, one dose right away, and additional doses on the third, seventh and fourteenth days.
They should also get another shot called Rabies Immune Globulin at the same time as the first dose, says the CDC.
This is because HRIG can partially suppress the active production of antibody.
The Human Rabies Immune Globulin, KEDRAB is a new human rabies immune globulin product in the United States approved by FDA in August 2017.
If you are traveling to one of the at-risk countries, the CDC has detailed information.
Recent rabies news:
Our Trust Standards: Medical Advisory Committee