Chikungunya Outbreaks Could be 60 Times More Fatal
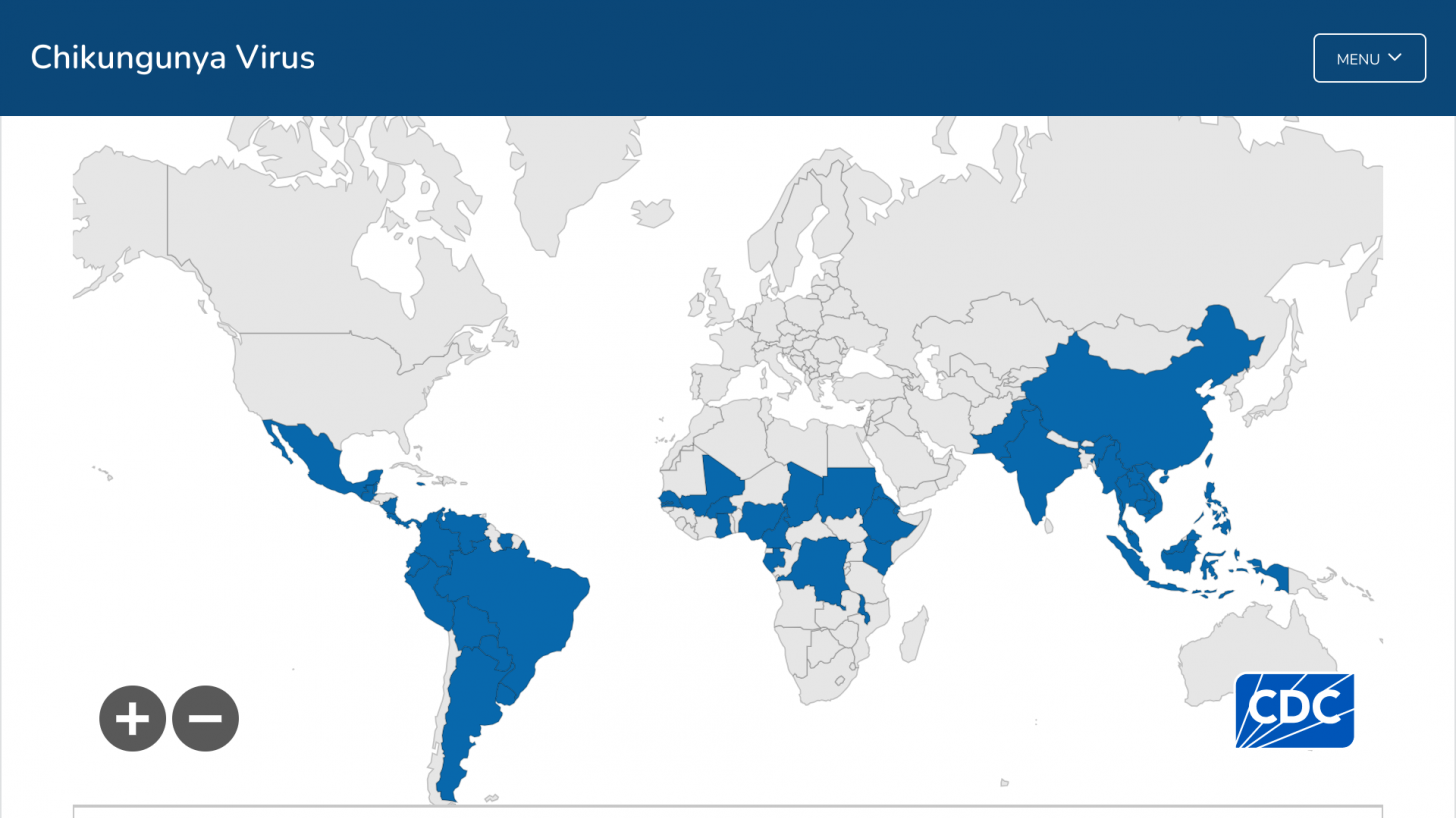
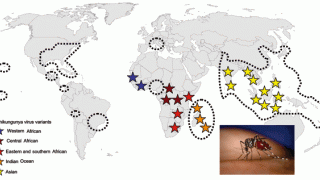
While the chikungunya virus (CHIKV) was first detected in 2014, its measurable impact on public health is just now being recognized in over 100 countries.
According to a Brief Research Report published by Frontiers in Tropical Disease on October 28, 2024, a significant chikungunya outbreak occurred in Minas Gerais, one of the most populous states in Brazil. These researchers analyzed the North and Northeast Health Macroregions of Minas Gerais, with 2.5 million inhabitants.
During this CHIKV epidemic, there were 890 excess deaths attributed to chikungunya, translating into a mortality rate of 35.1/100,000 inhabitants.
The excess mortality rate was 60 times higher than the deaths reported by surveillance, with only 15 confirmed deaths.
The correlation between excess deaths and laboratory-confirmed chikungunya cases was strong, while the correlation with dengue was not statistically significant.
These study results highlighted the severe underestimation of chikungunya mortality by epidemiological surveillance throughout the Region of the Americas.
During the same year, only 420 chikungunya deaths were reported by all Pan American Health Organization (PAHO) member countries.
In 2024, the PAHO reported over 404,372 CHIKV cases, but only 185 related fatalities in the Americas.
These researchers concluded that 'routine epidemiological surveillance systems cannot capture the full impact of this disease. Excess mortality is a key measure for understanding the impact of epidemics. The study highlights the need for complementary tools to traditional surveillance to assess better impacts on morbidity and mortality and support priority setting in public health interventions.'
Like many vaccines, CHIKV vaccine technologies include live-attenuated virus vaccines, inactivated viral vaccines, recombinant viral vaccines, chimeric-alphavirus candidates, DNA vaccines, and virus-like particles.
As of October 30, 2024, only Valneva SE's IXCHIQ® live-attenuated vaccine has been approved by the U.S. FDA.
IXCHIQ® is the first monovalent, single-dose, vaccine approved to prevent CHIKV infections in adults at increased risk of exposure to this mosquito-transmitted disease.
Our Trust Standards: Medical Advisory Committee