Eastern Equine Encephalitis Virus Vaccine for Humans Needed in the Northeast
The Massachusetts Department of Public Health (DPH) and the Massachusetts Department of Agricultural Resources (MDAR) recently announced plans to conduct aerial and truck-mounted spraying in parts of Worcester County at risk for the Eastern Equine Encephalitis (EEE) virus.
EEE is a rare but severe and potentially fatal mosquito-transmitted disease that can affect anyone.
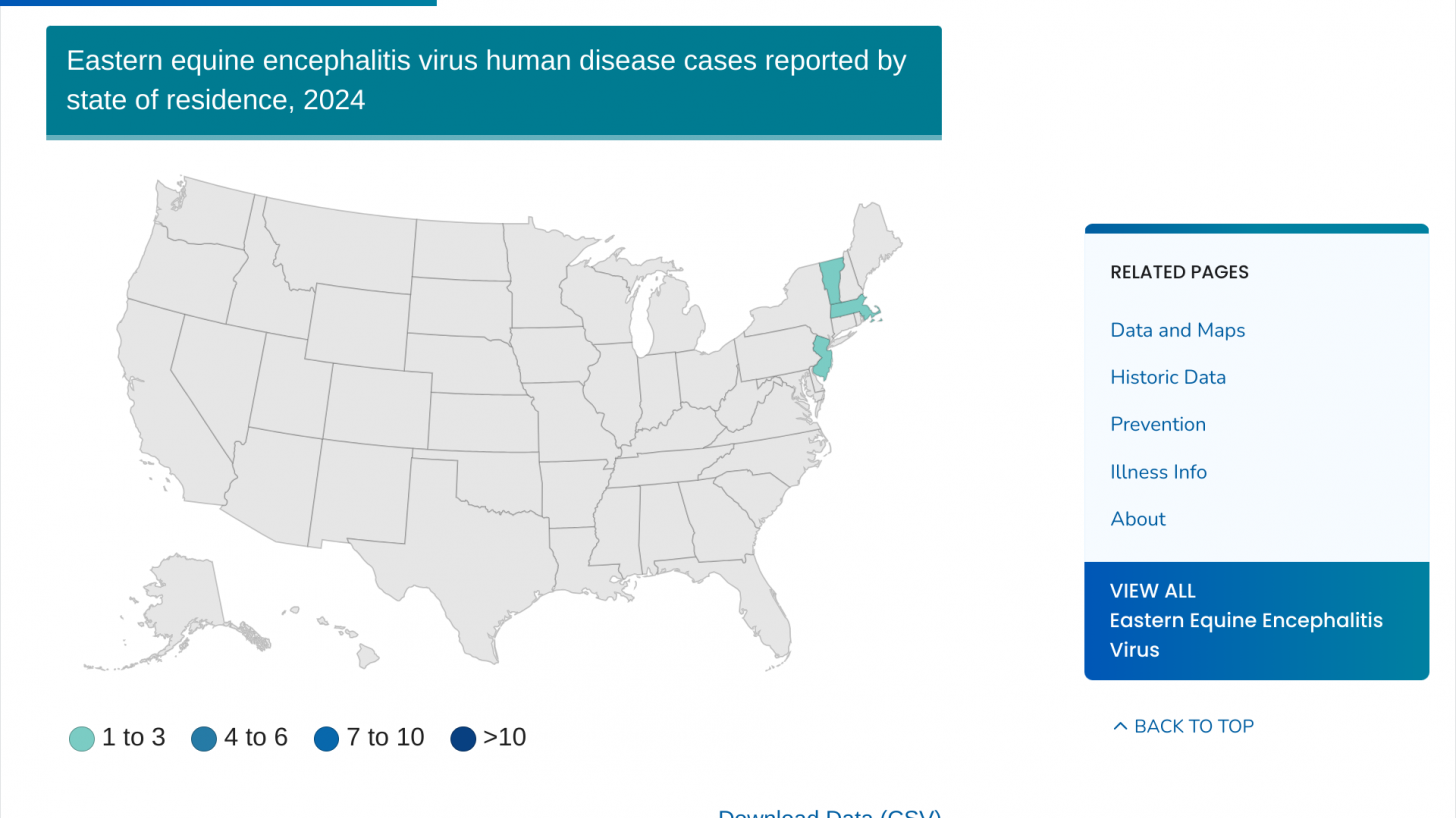
On August 16, 2024, DPH announced this year’s first human case of EEE virus infection in Worcester County.
As of August 24, 2024, the DPH has raised the risk of the EEE virus in ten communities in Massachusetts to high or critical. During the last EEE outbreak
In 2019, 38 confirmed EEE virus cases and 15 related deaths occurred throughout the northeastern states. Massachusetts reported 17 human cases and seven deaths.
DPH says the details on the exact timing will be provided as soon as possible, but spraying is expected to occur during the week of August 26. Spraying is scheduled at night, starting shortly after dusk and ending in the early morning. Please note that this schedule is weather-dependent and may change on short notice.
Massachusetts residents can visit DPH’s website for further information as it is made available.
Seperately, on August 26, 2024, New York State Agriculture Commissioner Richard A. Ball urged horse owners across New York State to vaccinate their horses against EEE and West Nile Virus, both of which are caused by a virus spread through the bite of an infected mosquito.
In 2024, New York has already seen cases of EEE found in horses in Clinton, Franklin, Saint Lawrence, Washington, Madison, Oneida, Orange, Ulster, Cayuga, and Wayne counties.
No confirmed cases of EEE in humans in New York this year exist.
Unfortunately, no U.S. FDA-approved EEE virus vaccines are available.
However, in May 2022, results from a Phase 1 clinical trial of a trivalent vaccine candidate developed by scientists at the National Institute of Allergy and Infectious Diseases Vaccine Research Center were published in The Lancet Infectious Diseases.
These researchers designed a virus-like particle (VLP) vaccine candidate (WEVEE) that uses proteins from the outer shells of the EEE, WEE, and VEE viruses to prompt an immune response. VLPs do not contain the genetic material the viruses need to replicate inside cells, so they cannot cause infection.
This study found the favorable safety profile and neutralizing antibody responses, along with pressing public health needs, support further evaluation of the WEVEE VLP vaccine in advanced-phase clinical trials.
Following this study, Emergent BioSolutions in Gaithersburg, Maryland, was issued a commercialization license for the advanced development of this vaccine candidate.
Our Trust Standards: Medical Advisory Committee




