Enhanced Recombinant BCG Vaccine Awaits FDA Approval

While the standard Bacillus Calmette-Guerin (sBCG) vaccine has been in use for about 100 years and administered over 4 billion times, its clinical efficacy is debatable.
On May 2, 2024, the Serum Institute of India (SII) confirmed that it is developing a new recombinant BCG vaccine (iBCG) to enhance efficacy and address the current supply shortages.
Founded in 1966, SII, the world's largest vaccine producer, has implemented two gene modifications to improve sBCG's immunogenicity and safety, creating iBCG.
The next-generation recombinant iBCG has completed Phase1/2 human clinical studies in Europe.
According to SII's press release, iBCG has demonstrated potent immunogenicity with CD8+ and CD4+ stimulation and improved safety compared to standard BCG in European clinical trials.
Furthermore, this iBCG vaccine should provide a long-term solution for the recently approved bladder cancer vaccine.
And iBCG is intended for use with ImmunityBio's ANKTIVA® (nogapendekin alfa inbakicept-pmln) for currently approved non-muscle invasive bladder cancer (NMIBC) and potential future indications.
"The collaboration between SII and Immunity Bio will undoubtedly transform how we approach cancer treatment. It will improve global access to BCG, and at the same time, this unique therapy is the key to achieving a complete solution for bladder cancer. We are truly excited to witness the incredible impact this collaboration will have on improving patient outcomes and saving countless lives," said Mr. Adar C. Poonawalla, CEO of SII, in a press release.
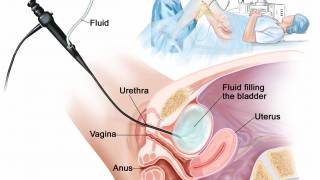
Originally used as a tuberculosis vaccine, BCG administered via intravesical instillation (delivery to the bladder via a catheter) has been the standard of care for patients with NMIBCr since 1977. BCG is a benign bacterium that induces an immune response in the bladder near the cancer cells, leading to cancer clearance in many patients.
"The scale and quality of vaccines that the Serum Institute manufacturers are unparalleled, and we are honored to partner with Dr. Poonawalla and his leadership team on this important initiative," added Richard Adcock, President and CEO of ImmunityBio.
"By providing another option for BCG, we believe more NMIBC patients will benefit from this proven treatment as both a monotherapy and a combination therapy with ANTKIVA."
ImmunityBio plans to conduct clinical trials to study iBCG and sBCG manufactured by SII in combination with ANKTIVA to treat different types of bladder and other cancers.
The supply of BCG is expected to be available once the protocol for the trial has been authorized by the U.S. FDA. ImmunityBio plans to submit the protocol to the FDA and global regulatory bodies in the next 30 days.
Merck & Co. is currently the only manufacturer of the BCG TICE® vaccine approved for use in the U.S. to prevent tuberculosis and treat certain bladder cancer patients. BCG is limitedly available in the U.S.
Globally, about 16 versions of BCG are in use to prevent tuberculosis in 2024.
Our Trust Standards: Medical Advisory Committee