Flu Vaccine Candidate Demonstrated Multi-Seasonal, Seven-Year Drifted Influenza Strain Protection

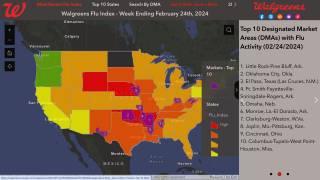
Influenza H3N2 virus continues to be a major cause of morbidity and mortality, especially for the older age groups, stated researchers in a new study published by The Journal of Infectious Diseases.
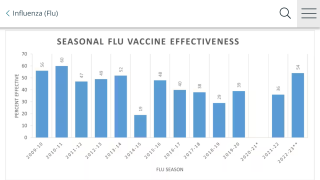
Although seasonal influenza vaccines are evaluated annually for the need to update the immunization with antigenically contemporary strains, H3N2 vaccine efficacy (VE) with currently licensed vaccines continues to be lower than for H1N1 or B viruses.
This void highlights the need for flu vaccines with broad-spectrum protection, stated these researchers on July 29, 2021.
In this human challenge study, they show that the intranasal M2SR vaccine candidate expressing HA and NA antigens that circulated more than a decade ago (2007) can protect against challenge with a highly drifted more contemporary H3N2 virus (2015) in individuals who demonstrate a cross-reactive serum response to the vaccine.
The magnitude of drift (HAI titer > 64 fold) between the vaccine and challenge virus was substantially more significant than that seen in a typical annual mismatch year (≥4 fold HAI differences).
After the challenge with drifted H3N2 virus, protection from infection and influenza illness was most prominent in the subset of M2SR recipients who demonstrated vaccine-induced pre-challenge increases in MNT to the challenge strain.
This M2SR vaccine group subset demonstrated a 46% reduction in infection and a 53% reduction in infection and illness than the placebo cohort.
In addition, this subset demonstrated overall reduced virus shedding and reduced clinical symptoms relative to placebo.
Moreover, this M2SR subset did not have any lower respiratory tract symptoms such as cough compared to the placebo cohort.
The intranasal M2SR vaccine-elicited serum and mucosal responses in addition to cellular responses demonstrating that M2SR generates a multi-effector immune reaction.
Serum and mucosal antibody responses were observed against both the vaccine and the challenge strain, demonstrating the potential of M2SR to generate broad-spectrum crossreactive responses.
‘We believe this is the first human challenge study to demonstrate protection against challenge with an influenza strain that has such a substantial antigenic difference from the vaccine strain and indicates the potential for M2SR to provide an improved breadth of protection compared to currently licensed vaccines,’ concluded these researchers.
This study also identified a potential serum marker of protection for the intranasal M2SR vaccine.
The mild AE profile of the M2SR vaccine indicates the potential for higher vaccine dose levels to provide further enhancements of protection by M2SR against highly drifted H3N2 influenza strains.
In this study, intranasal administration of 108 TCID50 of M2SR Bris2007 was well-tolerated, confirming previous observations of this intranasal, single replication virus vaccine. In addition, adverse events after dosing were mild, with no significant differentiation of vaccine from placebo.
Furthermore, there were no safety concerns related to the challenge with the influenza virus in individuals vaccinated with M2SR.
“Current vaccines are strain-specific, and in recent years they have had low efficacy against H3N2 influenza, especially when the vaccine is mismatched to circulating virus,” stated Dr. Robert Belshe, the Diana and J. Joseph Adorjan Endowed Professor of Infectious Diseases and Immunology, Emeritus, at Saint Louis University, and Chair of the FluGen Clinical Advisory Board, in a press release issued on August 2, 2021.
“The M2SR vaccine candidate is designed to induce a broad, multi-effector immune response, and this study demonstrated that subjects with vaccine-induced neutralizing antibodies were protected against infection and illness following challenge with an antigenically drifted virus. Inactivated vaccines have not had that broad protection.”
“This is the first demonstration in adults of vaccine-induced protection against a highly drifted H3N2 challenge virus.”
Redeeflu (M2SR) is a nasal vaccine candidate utilizing a proprietary M2 deleted, single replication (M2SR) influenza virus. M2SR, a supra-seasonal, live, single-replication, intranasal flu vaccine, says FluGen Inc.
The M2 gene is essential for the influenza virus to spread in the patient, and the deletion of the M2 gene restricts the virus to a single replication cycle in the host.
The body recognizes M2SR as an influenza infection. It activates its robust immune response, but because the virus can replicate only once, it cannot spread to other cells and cause symptoms of actual influenza infection.
Wisconsin-based Flugen Inc. is dedicated to improving public health by developing a vaccine that can prevent disease caused by all forms of the deadly influenza virus.
Note: The study was supported by a $15.4 million grant from the Department of Defense. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. And various industry relationships were disclosed by the researchers.
PrecisionVaccinations publishes fact-checked research-based vaccine news.
Our Trust Standards: Medical Advisory Committee