Fungal Disease Vaccines
Fungal Disease Vaccines
According to the World Health Organization (WHO), there is an urgent, need for reproducible and comparable estimates of severe fungal infections to address this often underappreciated public health need. The WHO published a report on October 25, 2022, highlighting the first-ever list of fungal priority pathogens, a catalog of the nineteen fungi representing the greatest threat to public health.
As of 2024, no fungal vaccines to protect people from disease have been approved by the European Medicines Agency (EMA) or the U.S. Food and Drug Administration (FDA). In mouse clinical studies, protection has been achieved with vaccine candidates directed against fungal pathogens, including species of Candida, Cryptococcus, and Aspergillus that most commonly cause life-threatening human disease. The U.S. Centers for Disease Control and Prevention (CDC) says There are millions of fungal species, but only a few hundred can make people sick. On January 11, 2024, the CDC published an MMWR stating that the large volume of topical antifungal prescriptions (6.5 million in 2023) in the context of emerging resistance highlights the need to better understand current prescribing practices, encourage judicious prescribing by clinicians, and improve patient education about recommended use.
Fungal Disease Vaccine Candidates
Fungal candidate vaccines target common antigens expressed in multiple genera of fungi, thereby protecting against a broad range of mycoses. Encouragingly, three vaccines have reached human clinical trials. Fungal vaccine candidates in 2023 are segmented into several broad categories based upon their composition, ranging from multiple to single antigens: whole organism vaccines (live-attenuated or killed fungal cells), crude extracts (fractions derived from cells and medium of fungal cultures), purified subunit vaccines (proteins, peptides), and nucleic acids (RNA and DNA) encoding the antigen(s) of interest.
A "pan-fungal" peptide NXT-2 vaccine is based on a previously identified vaccine candidate (Mar. 2022) and homologous sequences from Pneumocystis, Aspergillus, Candida, and Cryptococcus. Data in multiple animal models support the University of Georgia's concept that immunization with a pan-fungal vaccine before immunosuppression induces broad, cross-protective antifungal immunity in at-risk individuals.
Recombinant Candida vaccine candidates have reached human clinical testing with promising results. The PEV7 vaccine candidate consists of recombinant aspartyl-proteinase 2 (Sap2), a secreted protein of C. albicans, assembled into virosomes. After protection was demonstrated in C. albicans-challenged rats, a phase 1 clinical trial was conducted to assess the safety and immunogenicity of PEV7 in healthy female volunteers. All of the 48 vaccinated women developed specific B-cell memory responses.
The NDV-3A vaccine candidate contains the recombinant N-terminus of C. albicans agglutinin-like sequence three protein (Als3p, a cell surface adhesin, and invasin) formulated with aluminum hydroxide adjuvant. Preclinical studies demonstrated the vaccine was immunogenic and protected mice from Candida species. In the phase 1 clinical trial, which recruited 40 volunteers, NDV-3 elicited increased antigen-specific IgG and IgA1 titers and increased IFN-γ and IL-17A cytokine production compared to placebo recipients. Based on these data and a favorable safety profile, a multicenter, double-blind placebo-controlled phase 1b/2a clinical trial was undertaken to assess the immunogenicity and efficacy of the NDV-3A vaccine in 188 women with recurrent vulvovaginal candidiasis (RVVC)43. NDV-3A is identical to NDV-3, except it lacks a 6-His tag and linker sequences. As with NDV-3, NDV-3A was safe and highly immunogenic. There were no statistically significant differences between the treatment and placebo groups in the primary efficacy analysis. However, in a post-hoc subgroup analysis, subjects aged <40 had significantly fewer RVVC episodes during the 12-month study period.
Fungal Disease Outbreaks
The U.S. CDC's Mycotic Diseases Branch has assisted with dozens of fungal outbreak investigations. Invasive fungal infections cause over 1.5 million deaths worldwide. As of 2024, updated estimates suggest an annual incidence of 6.5 million invasive fungal infections and 3.8 million deaths, of which about 68% were directly attributable to fungal disease incidence. The CDC publishes a list of the most common Types of Fungal Diseases:
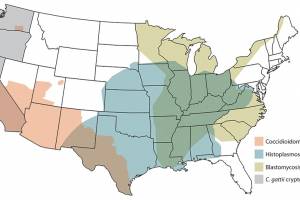
Valley fever - Currently, there is no vaccine to prevent Valley fever. Approximately 200 coccidioidomycoses (cocci) are associated with fatalities annually in the U.S., primarily in California and Arizona. Therefore, the U.S. NIAID awarded $4.5 million to establish Coccidioidomycosis Collaborative Research Centers to conduct vaccine research for this Valley Fever.
Candida auris is an emerging fungus that presents a serious global health threat. Research published in November 2022 concluded that a vaccine candidate could potentially treat C. auris infection.
The histoplasmosis outbreak occurred in campers in Louisiana in 2018.
Fungal meningitis and other fungal infections associated with contaminated steroid injections, 2012.
Fungal Disease Overview
The Annals of Internal Medicine reported on March 21, 2023, that fungus infections have risen dramatically since being detected in 2016. The increase in echinocandin-resistant cases and evidence of transmission is particularly concerning because echinocandins are first-line therapy for invasive Candida infections, including C auris. Invasive fungal infections cause over 1.5 million deaths worldwide. With the increased prevalence (~12%) of immunocompromised persons, invasive fungal infections have become considerably more frequent. High mortality rates caused by invasive mycoses and high morbidity because of intractable mucosal infections have created an unmet need for innovative prophylactic and therapeutic vaccine strategies against fungal pathogens.
Fungal Disease Vaccine News
September 30, 2023 - The Atlantic published an article: Humans Can No Longer Ignore the Threat of Fungi.
April 11, 2023 - NBCNews reported Michigan is investigating over 90 suspected cases of a potentially severe fungal infection linked to a Michigan paper mill. The Michigan Department of Health and Human Services, Surveillance for Healthcare-Associated and Resistant Pathogens Unit publishes updates on the emergence of Candida auris.
March 31, 2023 - A mycologist in Kolkata, Inda, is the first person to be diagnosed with a fungal disease that usually affects plants. This case report (June 2023) demonstrates plant pathogens can infect humans when in close contact with plant fungi.
March 22, 2023 - Cidara Therapeutics, Inc. announced that the U.S. FDA approved REZZAYO™ (rezafungin for injection) for treating candidemia and invasive candidiasis in adults with limited or no alternative treatment options.
March 16, 2023 - Researchers at the Department of Botany, Banaras Hindu University, and other institutions have discovered three unique genus of fungi.
February 6, 2023 - The journal Nature published The WHO fungal priority pathogens list as a game-changer.
January 31, 2023 - A vaccine candidate from the University of Georgia could be the first clinically approved immunization to protect people against invasive fungal infections.