Herpes Treatments
Herpes Treatments 2025
The U.S. Centers for Disease Control and Prevention (CDC) says there is no cure for herpes simplex virus (HSV) as of March 2025. However, the use of antiviral medicine shortens herpes outbreaks. According to Technavio, there were about 15 vendors in the herpes treatment market. To advance research to understand and address HSV infection, the U.S. NIH has established the Strategic Plan for Herpes Simplex Virus Research 2023-2028. This plan aligns with ongoing national efforts, including the Sexually Transmitted Infections National Strategic Plan. The CDC's STI Surveillance and Data Science Branch determined that the cost of each neonatal herpes patient is about $100,000.
Acyclovir is U.S. FDA-approved to decrease pain and speed the healing of sores or blisters in people with varicella, herpes zoster, and first-time or repeat genital herpes outbreaks. Acyclovir is also sometimes used to prevent outbreaks of genital herpes in people who are infected with HSV. Acyclovir is in a class of antiviral medications called synthetic nucleoside analogs. However, Acyclovir (valacyclovir 500 mg) will not cure genital herpes and may not stop the spread of genital herpes to other people. The Acyclovir product segment reported a significant retail pharmacy market share (50.44%).
Zydus Cadila commercializes Acyclovir Ointment USP (US RLD Zovirax), 5%. This medication treats the first onset of genital herpes. Those with weakened immune systems can also use it to treat lip and skin herpes infections that are not life-threatening.
Aurobindo Pharma Ltd. offers Valtrex® Tablets with 500mg base and 1g base for adults. However, it does not cure HSV.
Cipla Ltd. offers antiviral medicines for viral infections, such as herpes labialis, herpes simplex, shingles, and genital herpes, called ACIVIR 200 DT and ACIVIR 400 DT tablets.
Herpes Treatment Candidates 2025
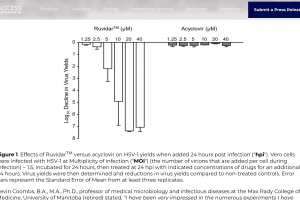
Ruvidar has been shown to deactivate various viruses effectively. The research was conducted at Kevin Coombs's Ph.D. laboratory at the University of Manitoba in conjunction with the National Microbiology Laboratory and Theralase® Technologies Inc. In animal studies, it may be more effective than Acyclovir.
AiCuris's Pritelivir (AIC316) is a potent inhibitor of HSV replication that belongs to a new chemical class and acts via a novel mechanism of action. In contrast to currently used nucleoside analogues, Pritelivir does not require activation by viral enzymes and can thus protect uninfected cells. On March 1, 2023, a clinical study suggested Pritelivir was safe and well tolerated, up to 600 mg following single and up to 200 mg following multiple once-daily doses. Considering a therapeutic dose of 100 mg once daily, pritelivir demonstrated a favorable safety, tolerability, and pharmacokinetic profile in healthy subjects to support further development. However, Pritelivir does not cure herpes.
Assembly Biosciences, Inc. announced the selection of development candidate ABI-5366 (5366) to progress to IND-enabling studies for its long-acting HSV-2 helicase inhibitor program.
Laboratoire Boreaderme Inc. and Ecogene 21 announced in September 2020 that they are focused on a Phase 2 clinical study to evaluate the safety profile of BOR15001L7 to docosanol 10% for managing cold sores in patients with recurrent herpes labialis.
A study published in May 2023 found that a molecule can effectively treat the lesions accompanying shingles and suggests the molecule may also work against the viruses that cause oral and genital herpes.
Herpes Encephalitis in Patients With Autoimmune Conditions or Immunomodulatory Medications
A large national population study published on May 2, 2024, found that Herpes virus encephalitis is strongly associated with preexisting autoimmune disease and exposure to immunosuppressive and immunomodulatory medications. To date, the role of antecedent immune-related dysregulation may have been underestimated.
Mothers' Antibodies Against HSV-1
A study published in the journal mBio® in 2017, headed by Dartmouth Researchers at Geisel, sheds new light on maternal antibodies' critical role in protecting neonatal nervous systems against infections. This study shows that antibody-secreting cells entered the trigeminal ganglion (TG), a key site of HSV infection, and persisted long after establishing latent infection. We also demonstrate the ability of passively administered IgG to enter the TG independently of infection, showing that the naive TG is accessible to antibodies.
Herpes Tests
The WHO announced in July 2023 that a new report on the Diagnostics Landscape for Sexually Transmitted Infections highlights diagnostics available to support scale-up of screening for STIs, including herpes. The U.S. Preventive Services Task Force published in February 2023 recommendations on screening for genital herpes infection in asymptomatic adults and adolescents, including pregnant individuals. Mylab Discovery Solutions in India launched the PathoDetect™ HSV Type 1& 2 Detection kit, a multiplex real-time PCR test that detects and differentiates HSV-l and HSV-2 in a single tube. And LabCorp Herpes Simplex Virus Types 1/2, DNA PCR.
Herpes Overview
Genital herpes is caused by herpes simplex virus type 1 (oral) and type 2, a common sexually transmitted disease (STD) associated with substantial health losses. To advance research to understand and address HSV infection, the U.S. National Institutes of Health (NIH), in September 2023, established the Strategic Plan for Herpes Simplex Virus Research 2023-2030. This plan aligns with ongoing national efforts, including the Sexually Transmitted Infections (STI) National Strategic Plan. A study published in 2023 estimated 0.05 lifetime quality-adjusted life years lost per incident infection, equivalent to losing 0.05 years or about 18 days of life for one person with perfect health.
A study published in October 2023 identified that increased serologic titers of herpes simplex virus 2 were associated with reduced whole-brain cortical thickness, and a combined score of HSV-2 and C. pneumoniae displayed an additive effect on reduced cortical thickness. These findings suggest HSV-2 seropositivity may contribute to accelerated brain aging, possibly resulting in an increased vulnerability to cognitive impairment and neurodegenerative disease in aging populations.
Herpes Vaccines
As of 2025, the U.S. FDA has not approved a herpes vaccine candidate.