Rabies Immune Globulin Reports Positive Study Results

Israel and New Jersey-based companies announced the results of their Phase 2/3 study for KEDRAB®, a human Rabies Immune Globulin.
In a press release on October 29, 2019, Kamada Ltd. and Kedrion Biopharma said the 2017 U.S. Food and Drug Administration (FDA) approval of KEDRAB for post-exposure prophylaxis (PEP) against rabies infection was based on the results of this study, which demonstrated that KEDRAB was non-inferior to the comparator HRIG product.
KEDRAB achieved Rabies Virus Neutralizing Antibody (RVNA) levels of ≥0.5 IU/mL on day 14 when each was co-administered with a rabies vaccine.
Additionally, KEDRAB was found to be well-tolerated during this study with a safety profile similar to that of the comparator HRIG product.
This is good news since there are only 2 licensed rabies immunoglobulin products available in the USA prior to the approval of KEDRAB.
“We are gratified that these important study results have now been published in a peer-reviewed medical journal, Human Vaccines & Immunotherapeutics,” said Amir London, Chief Executive Officer of Kamada, in this press release.
Furthermore, Kedrion and Kamada have recently completed the enrollment of 30 pediatric subjects in an FDA-required post-marketing trial in the U.S. with the primary objective of confirming the safety of KEDRAB in children aged 0 to 17 years.
The results of this study are expected in the second half of 2020.
Novinyo Amega, M.D., Kedrion Biopharma’s Head of U.S. Medical Affairs, added, “This trial, which is the first of its kind in this country, will expand on the data and research regarding safety and efficacy of HRIG in the pediatric population.”
KEDRAB [Rabies Immune Globulin (Human)] is a human rabies immunoglobulin (HRIG) indicated for passive, transient post-exposure prophylaxis (PEP) of rabies infection when given promptly after contact with a rabid or possibly rabid animal. KEDRAB should be administered concurrently with a full course of rabies vaccine.
The most frequent side events in subjects treated with KEDRAB in clinical trials were injection site pain, headache, muscle pain, and upper respiratory tract infection.
According to the World Health Organization, about 40 percent of exposures to suspect rabid animals occur in children under 15 years of age. But, there is currently very limited data on the safety and efficacy of any HRIG product in children.
Rabies is one of the oldest described infectious diseases, known for over 5,000 years. Rabies is a preventable, viral disease of mammals most often transmitted through the bite of a rabid animal.
Rabies is serious, and nearly always fatal, infection.
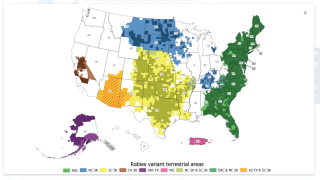
In the USA, rabies in wild animals, especially raccoons, skunks, foxes, and bats, accounts for most cases of rabies passed on to humans, pets, and other domestic animals. An acute, progressive viral encephalomyelitis, rabies carries the highest case fatality rate of any conventional etiological agent.
Bats are responsible for about 70 percent of rabies deaths among people who are infected with the rabies virus in the USA, reports the Centers for Disease Control and Prevention (CDC).
But, when traveling abroad, rabid dogs are a leading cause of rabies cases.
Rabies vaccine news
- 1 To 2 Shot Rabies Vaccine Candidate Launches Clinical Study
- Texas Air-Drops 1 Million Rabies Vaccine Packets
KEDRAB is marketed in the U.S. by Kedrion, which completed a successful launch of the product in the U.S. in 2018. Kedrion Biopharma is a leading international biopharmaceutical company that specializes in the development, production, and distribution of plasma-derived therapeutic products for use in treating serious diseases, disorders, and conditions such as immune system deficiencies and coagulation disorders.
Kamada Ltd. is focused on plasma-derived protein therapeutics for orphan indications and has a commercial product portfolio and a late-stage product pipeline.
To report SUSPECTED ADVERSE REACTIONS, contact Kedrion Biopharma Inc. Customer Service (1-855-353-7466) in the United States.
Rabies Vaccine News published by Precision Vaccinations
Our Trust Standards: Medical Advisory Committee
- Safety and efficacy results of simulated post-exposure prophylaxis with human immune globulin (HRIG; KEDRAB) co-administered
- Kamada and Kedrion Biopharma Announce Publication of Results from the Registration Study of KEDRAB in Human Vaccines & Immunothe
- Phase II/III Study of the Safety and Effectiveness of HRIG With Co-administration of Active Rabies Vaccine in Healthy Subjects
- Post-marketing Study of KamRAB Administered as a Single Dose With Active Rabies Vaccine in Children Exposed to Rabies