Marburg Disease Vaccines
Marburg Vaccines 2025
Marburg virus disease (MVD), a member of the Filoviridae family of viruses, is a severe but rare viral disease that has infected humans since 1967. As of February 14, 2025, no approved MVD vaccines are available. The World Health Organization (WHO) published the Marburg virus (MARV) vaccine development landscape on February 13, 2023. On March 30, 2022, the WHO R&D Blueprint team defined the Strategic Agenda for Filovirus Research and Monitoring (AFIRM) to establish research priorities for developing vaccines targeting filovirus diseases during the next decade. On April 4, 2023, the WHO Technical Advisory Group summarized the evaluations and recommendations on the four Marburg vaccine candidates.
Marburg Vaccine Candidates
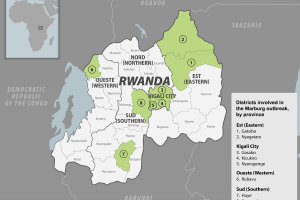
On October 5, 2024, Sabin announced it entered into a clinical trial agreement to provide 700 investigational doses for the PHV01 vaccine candidate to be dosed at six clinical trial sites in Rwanda. Pending a request from Rwandan officials and authorization from BARDA, Sabin plans to supply additional vaccines. Sabin's partner, ReiThera, has produced the drug substance and filled and finished doses for shipment to Rwanda. Public Health Vaccines, LLC launched its Phase 1 clinical trial (NCT06265012) in March 2024 to evaluate the safety and immunogenicity of its single-dose vaccine candidate, PHV01 (rVSV∆G-MARV-GP [Angola]), against the Marburg virus. The PHV01 vaccine is leveraging the proven recombinant vesicular stomatitis virus (rVSV) vector platform initially developed by the Public Health Agency of Canada. The U.S. Biomedical Advanced Research and Development Authority (BARDA) has funded PHV01.
Soligenix, Inc. MarVax™ is a subunit protein vaccine of recombinantly expressed MARV glycoprotein. On April 15, 2024, the U.S. FDA granted orphan drug designation to the active ingredient in MarVax™, the subunit protein vaccine of recombinantly expressed MARV glycoprotein, for "the prevention and post-exposure prophylaxis against MARV infection. On January 2, 2023, Soligenix, Inc. announced the journal Vaccine published a study describing the preclinical efficacy of a novel, single-vial, bivalent vaccine providing 100% protection against Sudan ebolavirus and Marburg marburgvirus infections. As of October 2024, the vaccines are being developed using the company's proprietary ThermoVax™ technology, which consists of a glycoprotein antigen from the viral surface of each virus that is manufactured in an S2 insect cell expression system and the CoVaccine HT™ adjuvant, which is known to stimulate both humoral (antibody) and cell-mediated (T cell) immunity. These are combined and lyophilized to ensure stability even at elevated temperatures (40ºC) for extended periods (at least two years). The vaccines are being developed with support from a U.S. NIH grant awarded to the University of Hawaii. A pre-IND meeting is expected in the next 12 months.
J&J Innovative Medicine and the NIAID launched a phase 1 study on August 9, 2016, evaluating AD26 FILO + MVA-BN-FILO's safety, Tolerability, and Immunogenicity of Heterologous Prime-boost Regimens Using the Multivalent Filovirus Vaccines Ad26.Filo and MVA-BN-Filo Administered in Different Sequences and Schedules in Healthy Adults.
IAVI's single-dose rVSVΔG-MARV-GP vaccine candidate against Marburg virus. Recently published preclinical data demonstrates that a single dose of the vaccine candidate is 100% efficacious at preventing MVD in nonhuman primates.
London School of Hygiene and Tropical Medicine—Ebola viruses, MARV GPs, and Tai Forest NP have been included in the modified vaccinia Ankara (MVA) vector vaccine (Ad26.ZEBOV, MVA-BN-Filo) in a Phase 2, open-label clinical trial that launched on March 17, 2022. This study evaluated the safety and immunogenicity of the 2-dose vaccination regimen in adults and children initially enrolled in the control arm of the EBOVAC-Salone study.
Researchers at the University of Oxford are developing the ChAdOx1 Marburg vaccine candidate in 2024. The Department of Health and Social Care funded the research as part of the U.K. Vaccine Network.
GeoVax Inc. vaccine candidate GEO-MM01 against the Marburg virus conferred 80% survival in cynomolgus macaques following a lethal dose of the Marburg virus on April 5, 2023. Vaccination protected nonhuman primates from viremia, weight loss, and death following challenges with a lethal Marburg virus dose. Evaluation of immune responses following immunization demonstrated the presence of both neutralizing antibodies and functional T cells, indicating a breadth of responses that combine for optimal protection.
In January 2023, the University of Texas Medical Branch in Galveston was awarded nearly $25 million from the.. government to develop vaccines to protect against infection, including Marburg.
On October 27, 2021, Orgininal Research demonstrated that the VSV-MARV is a fast-acting vaccine suitable for use in emergencies like disease outbreaks in Africa. Furthermore, data published on February 10, 2023, highlights VSV-MARV as a viable and fast-acting MARV vaccine candidate, supports the administration of a single low-dose vaccine in an emergency outbreak, and decreases the chances of vaccine-induced adverse events.
Marburg Outbreaks
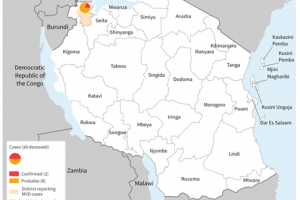
As of 2025, various African countries have reported Marburg disease cases, deaths, and outbreaks since 1967.