Chikungunya Vaccine Candidate Launches Phase 1 Study

A clinical-stage biotechnology company announced the publication of preclinical data showing that messenger RNA (mRNA) encoding a human monoclonal antibody against the chikungunya virus delivered in a proprietary lipid nanoparticle (LNP), can protect from infection by the virus in the living body of a plant or animal.
This preclinical data showed that treatment with mRNA was well tolerated at doses ranging from 0.3 mg/kg to 3.0 mg/kg in non-human primates, with linear dose-dependent pharmacology.
This means that increases in mRNA doses result in predictable and proportionate increases in expressed antibody in the blood.
Finally, in a mouse viral challenge model for chikungunya virus infection, the mRNA-encoded protein provided protection from arthritis, musculoskeletal tissue infection, and death.
Extrapolating these findings from the preclinical models suggests that persistence of antibody levels of at least 1 microgram per milliliter could be protective against chikungunya virus infection in humans.
mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane or secreted proteins that can have a therapeutic or preventive benefit and have the potential to address a broad spectrum of diseases.
This is important news since there are currently no effective therapies or approved vaccines to treat or prevent chikungunya infection or disease.
The company, Moderna, Inc, says these preclinical data supported the initiation of a limited, Phase 1 study of mRNA-1944 against chikungunya virus in January 2019.
This study of 32 participants will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of escalating doses of mRNA-1944 via intravenous infusion in healthy adults.
Stephen Hoge, M.D., president at Moderna, said in a press release, “Using the body’s own machinery to produce antibodies against chikungunya by using mRNA may be a powerful way to combat the virus.”
mRNA-1944 encodes a fully human IgG antibody originally isolated from B cells of a patient with a prior history of potent immunity against chikungunya infection, a mosquito-borne virus.
It is composed of two mRNAs that encode the heavy and light chains of this anti-chikungunya antibody within Moderna’s proprietary LNP technology.
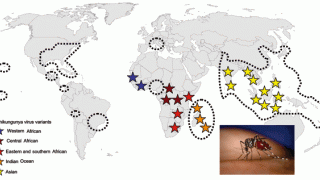
Chikungunya is a mosquito-borne virus that poses a significant public health problem in tropical and subtropical regions. The disease is characterized by an acute onset of fever, rash, muscle pain, and sometimes debilitating pain in multiple joints, says the Centers for Disease Control and Prevention (CDC).
Currently, people infected with chikungunya in the USA are treated with non-steroidal anti-inflammatory drugs to relieve some symptoms.
As of January 8, 2019, a total of 90 chikungunya virus disease cases with illness onset in 2018 have been reported from 23 U.S. states, says the CDC.
The research and development of mRNA-1944 was financially supported by the Defense Advanced Research Projects Agency, an agency of the U.S. Department of Defense, and the antibody was initially identified by researchers at Vanderbilt University Medical Center.
ModernaTX is developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, and cardiovascular diseases, independently and with strategic collaborators.
Our Trust Standards: Medical Advisory Committee
- Moderna Announces Publication of Preclinical Data for Chikungunya Antibody Program
- Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of mRNA-1944 in Healthy Adults.
- A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection
- Chikungunya Virus: Symptoms, Diagnosis, & Treatment