Monkeypox Outbreak Declared Health Emergency of International Concern
The WHO Director-General Tedros Adhanom Gebreyesus today transmitted the Report of the second meeting of the International Health Regulations Emergency Committee regarding the multi-country outbreak of the monkeypox virus (MPXV).
The Director-General declared a Public Health Emergency of International Concern (PHEIC) based on various inputs.
He stated today that not determining a PHEIC would not mean “business as usual.”
However, the WHO Committee Members were unable to reach a consensus regarding their advice to the Director-General regarding his PHEIC determination.
The Director-General also outlined the WHO response as of July 23, 2022, and the ongoing work to develop the WHO Strategic Readiness and Response Plan for monkeypox, being its overall goal to stop human-to-human transmission.
‘MPXV transmission is occurring in many countries that had not previously reported cases of monkeypox. And the current risk of monkeypox is moderate globally, except in the European region where the risk is high.’
For example, mathematical models estimate the basic reproduction number (R0) to be above 1 in Men-Sex-Men (MSM) populations and below 1 in other settings. In Spain’s urban centers, the estimated R0 is 1.8, in the United Kingdom 1.6, and in Portugal 1.4.
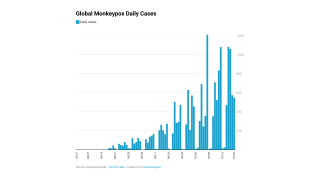
Globally, data sources indicate there are about 17,350 MPXV patients in over eighty countries, led by Spain and the USA, with 2,891.
And in New York City, 839 people have been infected between late May and July 22, 2022.
These data indicate a significant expansion in new MPXV cases over the past week.
The current clinical presentation of monkeypox occurring in outbreaks outside Africa is generally that of a self-limited disease, with rash lesions localized to the genital, perineal/perianal or peri-oral area, that often appears prior to the development of lymphadenopathy, fever, malaise, and pain associated with lesions says the WHO.
Moreover, investigations so far have not identified MPXv cases of occupational transmission, although investigations are ongoing.
The genome sequence of the virus obtained in several countries shows some divergence from the West African clade. Work is ongoing to understand whether the observed genomic changes lead to phenotypic changes, such as the reduced impact of countermeasures, vaccines, and treatments.
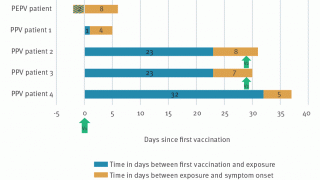
And the mean incubation period among cases reported is estimated at 7.6 to 9.2 days (based on surveillance data from the Netherlands, the U.K., and the USA).
Modeling work conducted by European Center for Disease Prevention and Control (ECDC) and the European Commission’s Health Emergency Preparedness and Response Authority (HERA) suggests that isolation of cases for 21 days and contact tracing could be effective in bringing the outbreak under control.
The modeling by ECDC and HERA is suggesting that the addition of vaccination-related interventions can increase the chances of controlling the outbreak, with pre-exposure prophylaxis of individuals at high risk of exposure appearing to be the most effective strategy to use vaccines when contact tracing is less effective, or impracticable.
However, the limited data on vaccine effectiveness against monkeypox constitutes one of the limitations of the modeling work conducted.
In the USA, the federal government recently confirmed it is aggressively expanding access to monkeypox vaccines (Jynneos) and treatments (TPOXX) from the Strategic National Stockpile (SNS).
On July 21, 2022, the U.S. HHS announced the government would have access to 786,000 additional government-owned doses that were physically inspected in Denmark during the week of July 4, 2022, and prepared for deployment by the end of July.
This announcement indicates more than 6.9 million Jynneos doses would be available by mid-2023.
Furthermore, prior to the start of the outbreak, the SNS held more than 1.7 million courses of TPOXX (tecovirimat) in its immediate holdings.
These treatments have and continue to be freely available to states and territories.
And since the CDC has worked with the FDA to clarify and simplify the process for accessing TPOXX, streamlining post-administration monitoring and data requirements.
And make clear to providers that the government’s documentation necessary to access TPOXX can be completed after it is prescribed to patients.
Other breaking news regarding the MPXV outbreak is posted at Vax-Before-Travel.
PrecisionVaccinations publishes fact-checked, research-based vaccine news curated for mobile readership.
Our Trust Standards: Medical Advisory Committee