Multinational Phase 2 Clinical Trial Launches Novel Immunotherapeutic for the Treatment of Chronic Hepatitis B

A Maryland-based biopharmaceutical company announced that it had enrolled the first patient in its multinational Phase 2 clinical trial of HepTcell, a novel peptide-based immunotherapeutic under development to treat chronic hepatitis B (CHB).
HepTcell is a novel immunotherapeutic composed of nine synthetic HBV-derived peptides formulated with IC31®, a TLR9-based adjuvant from Valneva SE. The HBV peptides are designed to drive T cell responses against all HBV genotypes in patients of diverse genetic backgrounds.
There is no cure for CHB, and currently, available antiviral medications only control the disease and require life-long treatment.
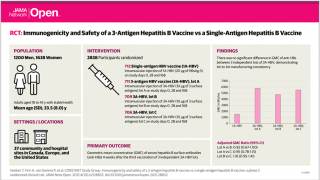
Altimmune, Inc. stated the study is being conducted in the USA, Canada, and Europe and is a double-blind, randomized, placebo-controlled study of 80 adult patients with HBeAg-negative inactive CHB and HBsAg ≤ 100 IU/mL.
HepTcell will be administered in 6 doses at 4-week intervals for 24 weeks, and patients will be followed for one year to evaluate the safety and durability of response.
“As one of the most common infectious diseases worldwide, there remains a significant unmet medical need for improved CHB therapies, as currently approved therapeutics prevent disease progression but rarely lead to a functional cure,” said Dr. Scott Harris, Chief Medical Officer of Altimmune, in a press release.
“We believe the T cell immune intolerance that characterizes the disease must be broken for the development of a functional cure. As a novel immunotherapeutic, HepTcell works by restoring dormant T cells to eliminate an infection.”
“While novel direct-acting agents under development, such as small inhibitory RNAs and capsid assembly modulators, have shown promise in reducing HBsAg load below 100 IU/mL, these agents have not resulted in the reactivation of T cell immunity and are unlikely to achieve durable virologic responses as monotherapies.”
“Based on the encouraging preclinical and clinical data, we are optimistic that HepTcell may be ideal in combination with novel direct-acting antivirals to achieve a functional cure for this disease.”
According to the World Health Organization estimates, CHB affects 292 million people worldwide, and nearly 900,000 people die annually of complications of the disease. If left untreated, CHB infection can lead to serious health issues, including cirrhosis, liver failure, and liver cancer.
Acute Hepatitis B infections are cleared through a T cell-dependent immune response. However, in chronically infected patients, high viral antigen load can induce a state of immune tolerance that prevents T cells from clearing the infection.
Restoring T cell function is considered essential to achieving a functional cure, defined as the loss of hepatitis B surface antigen (HBsAg) in the blood. Ultimately, all HBV therapeutics’ goal is to achieve a functional cure by reactivating the T cell immune response and overcoming immune tolerance.
In a Phase 1 clinical study of HepTcell conducted in the United Kingdom and South Korea, three monthly injections at two dose levels of HepTcell peptides were administered with and without IC31® adjuvant add-on therapy to entecavir or tenofovir in patients with Hepatitis B e-antigen (HBeAg)-harmful chronic infections.
In the Phase 1 study, all doses of HepTcell were generally well-tolerated. Both high and low doses of HepTcell given in combination with IC31® resulted in potent T cell responses against HBV antigens – representing a break in immune tolerance with no evidence of immune-mediated adverse events.
Altimmune is a clinical-stage biopharmaceutical company focused on developing intranasal vaccines, immune-modulating therapies, and liver disease treatments.
PrecisionVaccinations publishes research-based news.
Our Trust Standards: Medical Advisory Committee