New Evidence on Relative Effectiveness of Adjuvanted Seasonal Influenza Vaccine in Seniors

A worldwide leader in influenza prevention announced the publication of new real-world evidence (RWE) that demonstrated an MF59® adjuvanted, trivalent influenza vaccine (aTIV) was more effective than a standard-dose, non-adjuvanted trivalent seasonal vaccine in preventing influenza-related medical office visits and hospitalizations in seniors during the 2017-2018 influenza season in the USA.
Adding MF59® adjuvant to an influenza vaccine is designed to enhance the immune response to the influenza strains contained in the vaccine in adults 65 years and older.
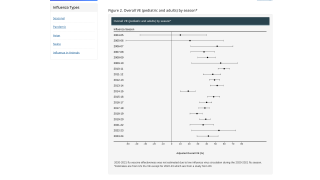
The Seqirus study was published in Vaccines on August 7, 2020, assessed relative influenza vaccine effectiveness (rVE) of aTIV compared to non-adjuvanted seasonal influenza vaccines including high-dose trivalent vaccine, standard-dose quadrivalent vaccine, and standard-dose trivalent vaccine.
The vaccines included in the economic burden analysis are the only influenza vaccines specifically indicated for patients 65 years and older.
The study also demonstrated that the effectiveness of aTIV and a high-dose trivalent influenza vaccine was comparable in the same population during the study season, emphasizing the critical role enhanced vaccines play in protecting adults 65 years and older against influenza.
This is the first Seqirus-sponsored study to conduct an economic burden analysis using a claims dataset comparing aTIV and a high-dose trivalent influenza vaccine.
The data revealed the all-cause and total influenza-related healthcare costs were comparable among older adults vaccinated with aTIV or a high-dose trivalent influenza vaccine during the 2017-2018 influenza season.
Additionally, participants who received aTIV had significantly lower rates of all-cause hospitalizations and pneumonia and asthma, COPD, bronchial hospitalizations, compared with TIV-SD.
Costs included those associated with all-cause total healthcare costs, influenza-related hospitalization costs, influenza-related ER costs, influenza-related office visits, and antiviral treatment costs, and influenza-related total costs.
Stephen I. Pelton, M.D., Professor of Pediatrics at Boston University School of Medicine and study author, stated: "The Centers for Disease Control (CDC) estimates that between 50 and 70 percent of seasonal influenza-related hospitalizations occurred in U.S. adults 65 and older.”
“Our data, published in Vaccines, demonstrate the important role enhanced vaccines contribute to the prevention of influenza in vulnerable populations and their potential to help reduce influenza-related morbidity and treatment costs."
Using data from IQVIA's Integrated Data Warehouse, which include Professional Fee Claims (Dx), Prescription Claims (Rx), and Hospital Charge Data Master (CDM), researchers studied adults 65 years and older who were vaccinated with a TIV, TIV-HD, non-adjuvanted, quadrivalent standard-dose influenza vaccine (QIV-SD), and non-adjuvanted, trivalent standard-dose influenza vaccine (TIV-SD) during the 2017/18 U.S. influenza season.
Adjusted rVE analysis indicated that aTIV was significantly more effective in preventing influenza-related medical office visits and influenza-related hospitalization/ER visits compared to QIV-SD and TIV-SD, and comparably effective to TIV-HD.
Influenza-related medical office visits were defined in the study based on a physician's office visit with a claim for a rapid influenza diagnostic test followed by antiviral treatment within two days of the test, and influenza-related hospitalization / ER visits were defined as patients with a diagnosis code for influenza.
Additionally, an economic analysis was conducted to compare influenza-related healthcare resource use and costs between patients that received aTIV and TIV-HD. These two vaccines were included in the analysis as they are the only influenza vaccines with specific indications for patients 65 years and older.
The analysis found that all-cause and influenza-related total costs were comparable between aTIV and TIV-HD.
This study was subject to the typical limitations associated with retrospective database studies.
Influenza is a common, contagious seasonal respiratory disease that may cause severe illness and life-threatening complications in some people. Influenza can lead to clinical symptoms varying from mild to moderate respiratory illness to severe complications, hospitalization, and in some cases, death.
Because transmission of influenza viruses to others may occur 1-day before symptoms develop and up to 5 to 7 days after becoming sick, the CDC recommends annual vaccination for individuals aged 6-months and older, who do not have any contraindications.
The CDC recommends that people get vaccinated by the end of October each year. However, getting vaccinated too early can be associated with reduced protection against influenza infection later in the flu season.
A listing of CDC authorized influenza vaccines for the 2020-2021 flu season in the USA, including Seqirus’ Fluad, is found on this PrecisionVaccinations webpage.
Seqirus is part of CSL Limited and is one of the largest influenza vaccine providers in the world.
PrecisionVaccinations publishes research-based flu vaccine news.
Our Trust Standards: Medical Advisory Committee