Rabies PrEP Vaccine Could Be Shortened by 33%

There may be a new, less complex methodology for rabies prevention.
A new study published in Clinical Infectious Diseases found that a reduced pre-exposure prophylaxis (PrEP) regimen for rabies was both effective and safe.
This is an important finding since rabies has a case fatality rate approaching 100 percent.
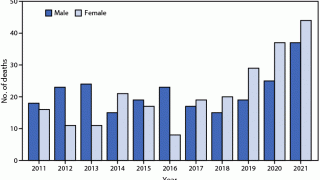
During 2015, the USA and Puerto Rico reported 5,508 cases of rabies in animals and 3 human rabies cases.
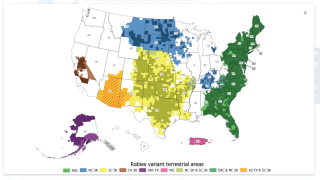
Rabies is a viral disease of mammals that is usually transmitted via the bite of a rabid animal. In the U.S., most rabies cases involve wild animals such as raccoons, skunks, bats, and foxes, says the Centers for Disease Control and Prevention (CDC).
In their study, Patrick Soentjens, MD, Ph.D., from the Institute of Tropical Medicine, Antwerp, Belgium, and colleagues investigated the safety and efficacy of using an ID vaccine, 2 doses, administered 7 days apart.
A total of 211 participants in the 2ID group and 200 in the 3ID group completed the primary vaccination regimens and received a booster vaccination.
This study reported a double dose of 0.1 ml of human diploid cell culture rabies vaccine (HDCV) over 2 visits was safe and not inferior to the single-dose 3 visit schedule.
Shortening the PrEp vaccination schedule is a positive change, since completing a 3 dose schedule is more time-consuming.
The standard PrEP schedule is comprised of 3 vaccinations given over a 28-day period. However, because this regimen is not always practical, effective, and safe, alternate schedules have been designed.
PrEP is a key component of rabies prevention and is recommended by the World Health Organization (WHO) for anyone at high risk of exposure to the virus because of their occupation, travel, or residence in a rabies-endemic area with poor access to timely and adequate PEP.
Rabies prevention involves two main strategies:
- dog vaccination to interrupt virus transmission to humans; and
- human vaccination as a series of vaccine administrations before an exposure or following an exposure.
Individuals with WHO category II or III exposures should receive PEP without delay.
The WHO rabies exposure categories are:
- Category I: Touching or feeding animals, licks on intact skin;
- Category II: Nibbling of uncovered skin, minor scratches or abrasions without bleeding;
- Category III: Single or multiple transdermal bites or scratches, contamination of mucous membrane or broken skin with saliva from animal licks, exposures due to direct contact with bats
PEP consists of the following steps:
- All bite wounds and scratches should be attended to as soon as possible after the exposure; thorough washing and flushing of the wound for approximately 15 minutes, with soap or detergent and copious amounts of water, is required. Where available, an iodine-containing, or similarly viricidal, topical preparation should be applied to the wound.
- RIG should be administered for severe category III exposures. Wounds that require suturing should be sutured loosely and only after RIG infiltration into the wound.
- A series of rabies vaccine injections should be administered promptly after an exposure.
Currently, rabies vaccines made from inactivated cell cultures are extremely well tolerated.
The full version of the WHO position on rabies vaccines and immunoglobulins is published in the Weekly Epidemiological Record April 2018.
Our Trust Standards: Medical Advisory Committee