Chikungunya Vaccine Candidate Reports Favorable Phase 2 Results

The chikungunya fever vaccine candidate (MV-CHIK) reported very positive results for a Phase 2 clinical trial.
This study met all endpoints across the treatment groups.
In this double-blind study, MV-CHIK induced neutralizing antibodies against Chikungunya in all treatment groups after 2 injections, with seroconversion rates ranging from 86.4 percent to 100.0 percent.
The trial also assessed the impact of pre-vaccination against the vector in 2 groups that received Priorix ®, the live attenuated measles, mumps and rubella (MMR) vaccine, prior to MV-CHIK administration.
The data showed that pre-existing antibodies against the measles vaccine virus did not affect immunogenicity against Chikungunya.
This clinical trial included 263 healthy participants and was conducted at 4 study sites in Austria and Germany.
“The recent addition of Chikungunya fever to the Tropical Disease Priority Review Voucher Program of the US Food and Drug Administration (FDA) is a clear indication that the development of a chikungunya vaccine is urgently needed,” commented Dr. Erich Tauber, CEO, and founder of Themis, in a press release.
MV-CHIK is the first candidate from Themis’ innovative immunomodulation platform based on the measles vector, one of the safest and most efficacious vaccines available.
MV-CHIK has already been tested in over 600 study volunteers in the US, EU, and Central America and was recently awarded PRIME designation status from the European Medicines Agency.
Emil Reisinger from Rostock University Medical Center and a co-principal investigator of the study said, “The outbreak prevalence has increased due to a higher frequency of travel and the lack of treatment options and thus remains a major public health concern.”
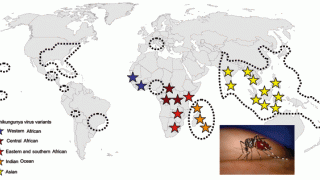
The chikungunya fever virus belonging to the Togaviridae family is transmitted to people through mosquito bites from Aedes aegypti and Aedes albopictus mosquitoes.
These mosquitoes bite people during the day and at night, says the FDA.
“We are highly committed to seeking approval to commence a pivotal Phase 3 trial in the near term,” said Dr. Tauber. For more information, visit Themis.
In late 2013, the first ‘local’ transmission of chikungunya virus in the Americas was identified in the Caribbean. Local transmission means that mosquitoes in the area have been infected with the virus and are spreading it to people.
Beginning in 2014, a chikungunya virus local transmission was identified in Florida, Texas, Puerto Rico, and the U.S. Virgin Islands.
Chikungunya has been identified by the World Health Organization (WHO) in over 60 countries in Asia, Africa, Europe, and the Americas.
As of October 2018, the US Centers for Disease Control and Prevention has not issued a chikungunya related Travel Alert.
Our Trust Standards: Medical Advisory Committee
- Themis Bioscience Publishes Compelling Phase 2 Results for Lead Vaccine Candidate against Chikungunya Fever in The Lancet
- Phase II Study to Evaluate Safety and Immunogenicity of a Chikungunya Vaccine (MV-CHIK-202)
- Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK
- FDA In Brief: FDA adds four tropical diseases to priority review voucher program to encourage drug development in areas of unmet
- Chikungunya Fever Vaccine Candidate Gains PRIME Designation
- Chikungunya Vaccine Candidate Advances into Phase 1 Second Stage