Vaccine Effective Against Various Meningococcal B Strains

Two clinical studies reported that the TRUMENBA vaccine, as a three-dose series, elicits a protective immune response against diverse meningococcal group B (MenB) strains.
The proportion of subjects that achieved a prespecified hSBA titer for all four primary strains, were 82.7% in adolescents, and 84.5% in young adults, after dose three of TRUMENBA.
These Phase 3 clinical studies, published in the New England Journal of Medicine, and were reported to have met all five co-primary immunogenicity endpoints.
The Centers for Disease Control and Prevention (CDC) recommends routine use of MenB vaccines among persons aged 10 years and older who are at increased risk.
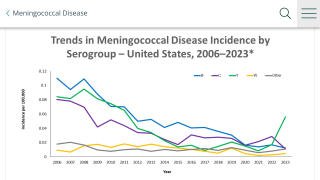
The majority (90%) of invasive meningococcal disease cases can be attributed to six N meningitidis serogroups (A, B, C, W, X, and Y), according to the CDC.
Recently updated CDC recommendations advise using the three-dose TRUMENBA schedule (0, 1‐2, and 6 months) in high-risk individuals, and the two-dose schedule (0 and 6 months) for healthy adolescents and young adults.
“MenB disease is uncommon, yet serious, is unpredictable and can strike at any age, including healthy teenagers and young adults in the prime of their lives, with potentially long-lasting and devastating consequences, including mortality,” said Federico Martinon-Torres, MD, Ph.D., head of Pediatrics and director of Translational Pediatrics and Infectious Diseases at Hospital Clínico Universitario de Santiago de Compostela, Spain.
Early meningococcal disease symptoms can be misinterpreted as the flu, often making it difficult to diagnose and delaying treatment.
The most common clinical presentations of meningococcal disease are meningitis and septicemia.
Despite prompt and appropriate antibiotic treatment, 10 to 15 percent of people with meningococcal disease will die, reports the CDC.
Of those adolescents who survive, 60 percent experience significant physical and mental disabilities, such as brain damage, hearing loss, learning disabilities, or limb amputations.
In these Phase 3 clinical studies, TRUMENBA, administered on a three-dose schedule (0, 2, and 6 months), demonstrated immunogenicity against four primary MenB strains representative of strains causing invasive disease.
Immune responses in both studies were assessed by serum bactericidal assays using human complement (hSBA). The primary immunogenicity objectives assessed the immune responses to four primary MenB test strains representative of prevalent MenB strains one month after dose three.
The secondary endpoint measured responses to 10 additional diverse, disease‐causing MenB test strains.
The responses to the 10 additional strains were comparable to those seen against the four primary strains. The most common solicited adverse reactions in adolescents and young adults were pain at injection site, fatigue, headache, and muscle pain.
TRUMENBA was granted Accelerated Approval in the U.S. in October 2014 for active immunization to prevent invasive disease caused by N meningitidis serogroup B (MenB) in individuals 10 through 25 years of age.
These Phase 3 data supported the transition to Traditional Approval in the U.S. for the three-dose schedule and were pivotal for approvals in Europe, Australia, and Canada earlier this year.
TRUMENBA can be administered as a two- or three-dose schedule depending on an individual’s risk of exposure and susceptibility to MenB.
Ask your healthcare provider about the risks and benefits of TRUMENBA. Only a healthcare provider can decide if TRUMENBA is right for you or your child.
Most pharmacies offer MenB vaccines.
The CDC Vaccine Price List provides the private sector vaccine prices for general information.
Meningococcal discounts can be found here.
Vaccines, like any medicine, can have side effects, says the CDC.
You are encouraged to report negative side effects of vaccines to the U.S. Food and Drug Administration (FDA) and the CDC.
Contact: [email protected]
Our Trust Standards: Medical Advisory Committee
- Meningococcal Vaccines for Preteens, Teens
- Meningococcal Disease
- A Bivalent Meningococcal B Vaccine in Adolescents and Young Adults
- Updated Recommendations for Use of MenB-FHbp Serogroup B Meningococcal Vaccine — Advisory Committee on Immunization Practices, 2
- PHASE 3 TRUMENBA® (MENINGOCOCCAL GROUP B VACCINE) DATA PUBLISHED IN NEW ENGLAND JOURNAL OF MEDICINE DEMONSTRATE THE VACCINE’S IM