Chikungunya Vaccine Candidate Reports 100% Seroconversion Rate

A single-shot vaccination of the Chikungunya vaccine candidate VLA1553 showed excellent immunogenicity and acceptable safety profiles, reported the French biotech company, Valneva SE.
This is important news since there is not a commercially approved vaccine to prevent, nor medicine to treat, the Chikungunya virus disease.
Previously, the U.S. Food and Drug Administration (FDA) granted Fast Track designation for VLA1553.
The interim results of the VLA1553-101 vaccine candidate phase 1 clinical trial showed a 100 percent seroconversion rate achieved at Day 28, in a pooled analysis of all vaccinated groups.
Additionally, this study’s results showed 96.5 percent of subjects achieved at least a 16-fold increase in antibody titers and a high geometric mean titer, fully supporting VLA1553′s differentiated target product profile.
The pooled safety profile of all groups was considered acceptable and supports further development. No serious adverse events nor adverse events of special interest were reported up to Day 28.
As with other live-attenuated vaccines, transient cases of reduced levels of neutrophils, lymphocytes or leucocytes, without clinical symptoms, were observed in the pooled analysis.
As of December 11, 2018, a total of 83 chikungunya cases with illness onset were reported in the USA, says the Centers for Disease Control and Prevention (CDC).
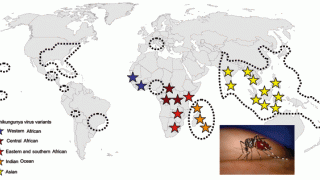
Since 2013, the Chikungunya virus has spread through the Americas, and as of December 2018, had been reported by 100 countries.
And during 2017, the Pan American Health Organization confirmed 123,087 Chikungunya cases in the Americas.
Wolfgang Bender, M.D., Ph.D., Chief Medical Officer of Valneva commented in a press release, “Phase 1 revaccination data post month 6 is expected by mid-year and will be key to define development acceleration options.”
Valneva is committed to advance its Chikungunya vaccine candidate as quickly as possible into pivotal trials, after dialogue and alignment with the authorities.
Chikungunya is a mosquito-borne viral disease caused by the Chikungunya virus, which is a Togaviridae virus.
Chikungunya is transmitted to humans by the bites of infected female mosquitoes. Most commonly, the mosquitoes involved are Aedes aegypti and Aedes albopictus, two species which can also transmit other mosquito-borne viruses, including dengue.
Chikungunya virus causes clinical illness in 72-92 percent of infected humans around 4 to 7 days after an infected mosquito bite.
This study is a randomized, observer-blinded, multicenter, dose-escalation Phase 1 clinical study investigating 3 dose levels of VLA1553 after a single immunization.
The study enrolled 120 healthy volunteers, 18 to 45 years of age, in the United States.
Additional information, including a detailed description of the study design, eligibility criteria, and investigator sites, is available at ClinicalTrials.gov.
Valneva SE is a biotech company developing and commercializing vaccines for infectious diseases with major unmet needs.
Our Trust Standards: Medical Advisory Committee
- Valneva Reports Positive Phase 1 Interim Results for Its Chikungunya Vaccine Candidate
- Study to Assess the Safety and Immunogenicity of a Chikungunya Virus Vaccine Candidate (VLA1553) in Healthy Volunteers
- Where Has Chikungunya Virus Been Found?
- Chikungunya Vaccine VLA1553 ‘Fast-Tracked’ By FDA
- Chikungunya Vaccine Candidate Advances into Phase 1 Second Stage