Monkeypox Vaccination Produced de Minimis Protection

When the monkeypox outbreak began in May 2022, most impacted people started a search for a preventive vaccine. In the U.S., the government began offering vaccinations in Boston, then in New York and Washington DC.
But without peer-reviewed clinical studies, vaccine efficacy remained in question.
A recent study published by the journal Nature Medicine found the antibody response to the two-dose JYNNEOS® (MVA-BN) vaccine in non-primed people or those who had not previously received a smallpox vaccine was very low.
Erasmus University Medical Center researchers in the Netherlands show that monkeypox virus (MPXV) neutralizing antibodies can be detected after MPXV infection and after historic smallpox vaccination.
However, a two-dose JYNNEOS immunization series in non-primed individuals yields relatively low MPXV-neutralizing antibodies.
And dose-sparing of an MVA-based influenza vaccine leads to lower MPXV-neutralizing antibody levels.
Whereas a third vaccination with the same MVA-based vaccine significantly boosts the antibody response.
Monkeypox-neutralizing antibodies were detected across individuals after infection.
Although the researchers detected monkeypox-reactive antibodies in only 5 of 19 monkeypox-infected individuals born after 1974.
'The role of MPXV-neutralizing antibodies as a correlate of protection against disease and transmissibility is currently unclear.'
'We conclude that cohort studies following vaccinated individuals are necessary to assess vaccine efficacy in at-risk populations,' wrote these researchers on October 18, 2022.
Bavarian Nordic's 3rd generation smallpox vaccine has been authorized by the U.S. FDA (2019) and is used as a vaccine against the MPXV.
In the U.S., the standard regimen is subcutaneous administration: a volume of 0.5mL, but the current U.S. CDC-recommended regimen is intradermal administration: an injection volume of 0.1mL.
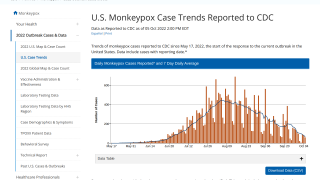
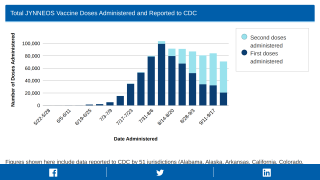
As of October 18, 2022, the CDC reported that 975,047 doses of JYNNEOS had been administered in 56 U.S. Jurisdictions Reporting.
And as of October 19, 2022, the U.S. government had distributed 844,793 doses.
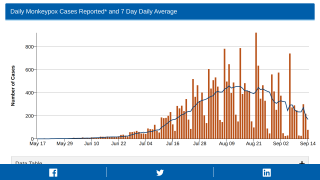
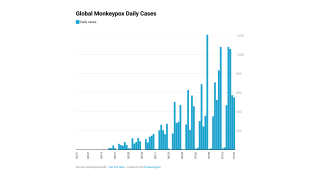
The CDC reported 27,635 total confirmed monkeypox/orthopoxvirus cases since May 2022.
Other monkeypox outbreak news and research are posted at Monkeypox Today.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually translated and curated for mobile readers.
Our Trust Standards: Medical Advisory Committee