More Mpox Vaccine Breakthrough Cases
Eurosurveillance recently published a Rapid Communication report focused on a case series of Belgian patients presenting with severe Mpox disease following post-exposure preventive vaccination (PEPV) or after off-label primary prophylactic vaccination (PPV) in non-primed individuals.
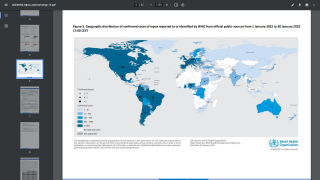
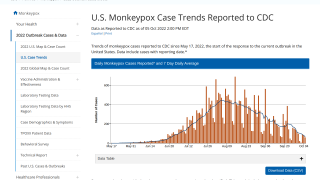
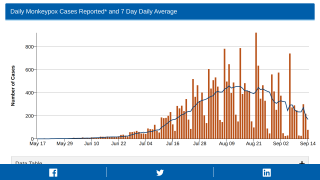
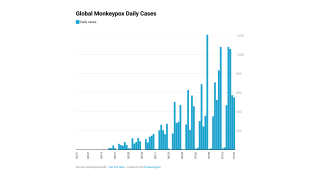
Thus far, the current control measures have positively impacted the Mpox outbreak, as indicated by the sharp decline in new infections.
In Belgium, the Mpox vaccination with the U.S. FDA-approved modified vaccinia Ankara (MVA-BN; JYNNEOS®, IMVANEX®) vaccine started with PEPV end of May 2022.
From the end of July, the main Belgian clinics began PPV campaigns by administering a first subcutaneous (SC) dose.
Without immunosuppression, the second dose was delayed instead of being given at the recommended interval of 28 days.
In agreement with recommendations from several health councils, vaccinations from September 2, 2022, onwards were exclusively given off-label via the intradermal route at one-fifth of the SC dose.
Since the end of November 2022, the vaccine supply was secured and was again given as two SC doses with an interval of 28 days.
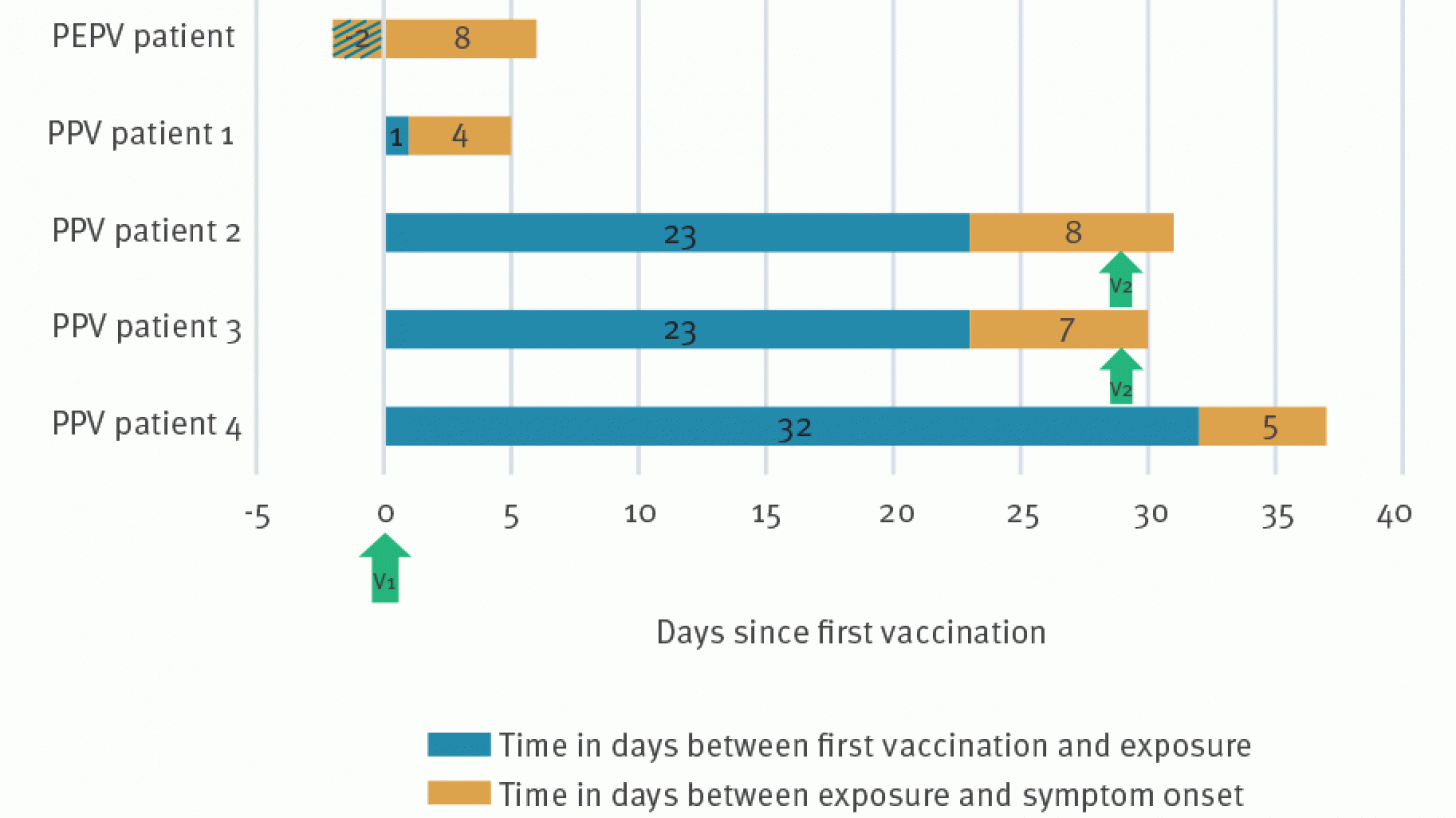
Three patients presented with symptoms compatible with mpox after vaccination at our institute between the end of July and the beginning of October. Two others were diagnosed with mpox at an emergency department.
They contacted the research team after the diagnosis in October.
All five patients had at least one full dose of the subcutaneous JYNNEOS vaccine before contracting the Mpox virus again.
These exposures were reported between two days before (PEPV patient) and 32 days after vaccination.
The rationale for different dosing options was based on older epidemiological studies demonstrating the cross-protection of first-generation smallpox vaccines against the Mpox virus.
However, the clinical efficacy of third-generation smallpox vaccines (JYNNEOS) against Mpox and established correlates of protection remain to be determined.
A few studies have been published thus far.
A non-profit healthcare provider in the United States recently reported 90 patients with Mpox among its 7,339 vaccinees, with most infections occurring in the first two weeks after the first dose.
Although two cases were infected > 14 days after the second dose.
Notably, eight individuals developed symptoms, including some with rectal pain and proctitis, more than 28 days after the first vaccine dose.
Five of these infections occurred after receiving a second dose.
A recent French study detected 12 (4%) PCR-confirmed mpox cases in 276 individuals who received PEPV.
They reported two proctitis cases that were classified as non-severe, as no hospitalization occurred.
These researchers concluded a uniform definition of Mpox disease severity would help to facilitate comparability across studies.
Nevertheless, healthcare workers and those at high risk should remain aware of the possibility of infections after vaccination, especially shortly after administering the first dose and be vigilant for symptoms.
Furthermore, combining Mpox vaccination with preventive measures should be emphasized.
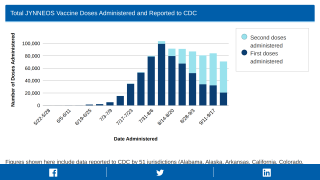
In the U.S., 1,118,639 JYNNEOS doses (first and second) have been administered in 57 Jurisdictions as of November 29, 2022.
Additional Mpox news is posted at MonkeypoxToday.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee