BA.2 Variant Knocks Out Sotrovimab in the U.S.

The U.S. FDA today announced the monoclonal antibody (mAbs) Sotrovimab is no longer authorized to treat COVID-19 in any U.S. region due to increases in the proportion of COVID-19 cases caused by the Omicron BA.2 sub-variant.
Data in the FDA health care provider fact sheet show that the authorized dose of sotrovimab is unlikely to be effective against the BA.2 sub-variant.
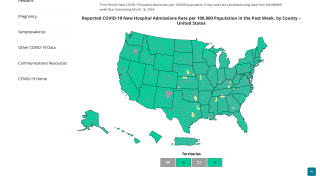
The Centers for Disease Control and Prevention Nowcast data from April 5, 2022, estimates that the proportion of COVID-19 cases caused by the Omicron BA.2 variant is above 50% in all Health and Human Services (HHS) U.S. regions.
The FDA confirmed it would continue to monitor BA.2 in all U.S. regions and provide follow-up communication when appropriate.
In Europe, where BA.2 is also prevalent, Sotrovimab (Xevudy) remains authorized as of April 5, 2022.
Additional mAbs breaking news is posted at PrecisionVaccinations.com/antibodies.
Note: The FDA announcement was edited for clarity and manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee