One STD is Decreasing As Off-Label Vaccine Use Increases
The U.S. CDC recently published a Sexually Transmitted Infections Surveillance Report for 2023, which provides the latest data on trends for nationally notifiable sexually transmitted infections (STIs), including gonorrhea.
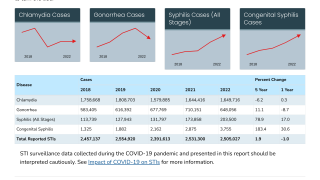
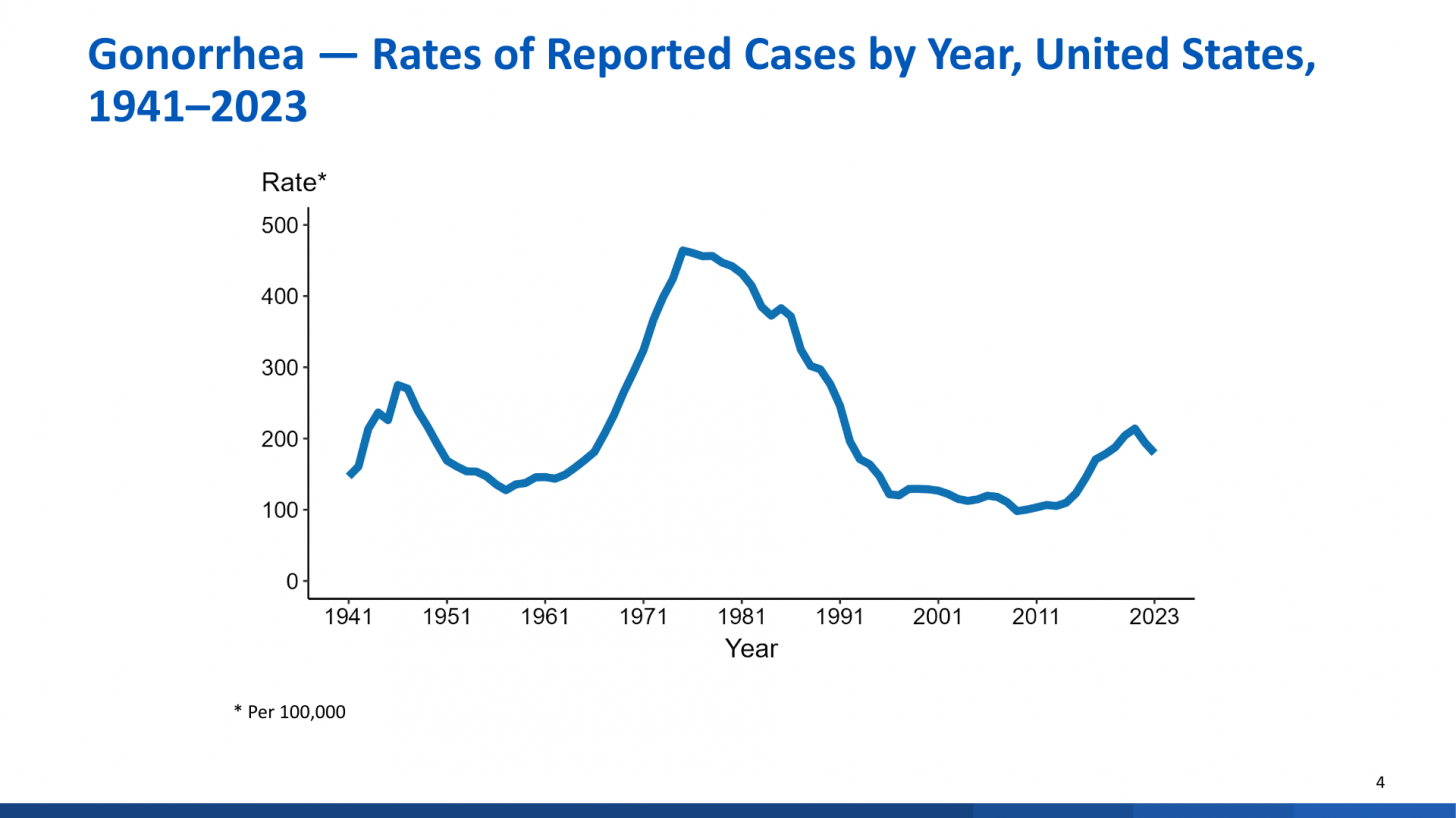
Published on November 13, 2024, this report revealed a total of 601,319 cases of gonorrhea were reported in 2023, making it the second most common nationally notifiable STI in the U.S.
This CDC report shared some good news.
The overall gonorrhea rate declined 7.7% from 2022 to 2023.
At present, the only CDC-recommended treatment of uncomplicated urogenital, anorectal, and pharyngeal gonorrhea is monotherapy with a single intramuscular dose of ceftriaxone 500 mg.
However, there is a U.S. FDA-approved vaccine has shown effectiveness against gonorrhea over the past few years.
Although a gonorrhea-specific vaccine does not exist, N. gonorrhoeae shares much of its genome with Neisseria meningitidis, notably critical antigenic determinants including outer membrane vesicles (OMV). The effectiveness of a meningococcal vaccine against gonorrhea stems from the fact that related bacteria cause the two diseases—Neisseria meningitidis and Neisseria gonorrhoeae, respectively.
In the U.S., GSK's Bexsero® (MenB-4C) vaccine is FDA-approved for intramuscular injection to prevent meningococcal group B disease caused by Neisseria meningitidis bacteria. As of 2024, people between 16 and 23 years of age can receive Bexsero based on 'shared clinical decision-making' and receive two doses six months apart.
Furthermore, real-world evidence revealed that Bexsero provides cross-protection against gonorrhea.
On July 8, 2024, the Journal of Infection published results from a systematic review and meta-analysis. Adjusted vaccine effectiveness (VE) for OMV vaccines against gonorrhea ranged from 22% to 46%. The pooled VE estimates of OMV vaccines against any gonorrhea infection following the entire vaccine series were 33-34%.
The study authors concluded that 4CMenB and other MenB-OMV vaccines show moderate effectiveness against gonorrhea.
GSK recently announced that meningitis vaccine sales grew double-digits for Q3 2024 and YTD, achieving record quarterly sales. Bexsero sales grew primarily due to U.S. CDC purchasing patterns, a favorable pricing mix in the U.S., a recommendation in Germany, and the launch in Vietnam.
In the U.K., the Joint Committee on Vaccination and Immunisation recommended, on November 10, 2023, a routine targeted vaccination program using Bexsero® to prevent gonorrhea for specific age groups.
Dr. Nick Phin, Director of Public Health Science at Public Health Scotland, said in a media release in November 2023, "The recent increase in gonorrhea cases, mainly in gay, bisexual, and other men who have sex with men, is very concerning and the offer of the 4CmenB vaccine to this group is a welcome new intervention to help control and prevent the spread of this infection."
In the U.S., Bexsero is offered at many health clinics and pharmacies, including on college campuses.
Our Trust Standards: Medical Advisory Committee
- National Overview of STIs in 2023
- Vaccine effectiveness and impact of meningococcal vaccines against gonococcal infections
- GSK - 2024 Third quarter results
- Prevention of Neisseria gonorrhoeae With Meningococcal B Vaccine: A Matched Cohort Study in Southern California
- The Serogroup B Meningococcal Vaccine Bexsero Elicits Antibodies to Neisseria gonorrhoeae