Bexsero MenB Vaccine May Offer Gonorrhea Protection

New research indicates that the meningococcal B vaccine Bexero may offer cross-protection against gonorrhea.
Bexsero, which is FDA-approved to prevent invasive disease caused by Neisseria meningitidis serogroup B, for individuals 10 to 25 years of age, includes the MeNZB OMV component plus 3 recombinant antigens, NadA, fHBP-GNA2091 and NHBA-GNA1030.
This research found there is a high level of sequence identity between MeNZB OMV and Bexsero OMV antigens, and gonococcal proteins in test rabbits.
This study’s conclusion said ‘the anti-gonococcal antibodies induced by MeNZB-like OMV proteins could explain the previously seen decrease in gonococcal cases following MeNZB vaccination.
And, the high level of anti-gonococcal-NHBA antibodies generated by Bexsero vaccination in humans may result in additional cross-protection against gonorrhea.
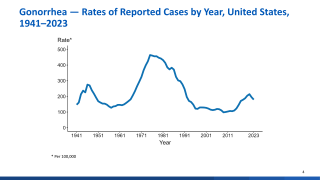
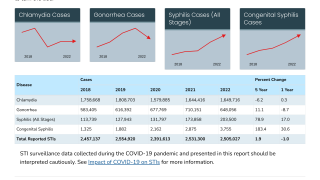
Gonorrhea is the 2nd most commonly reported notifiable disease in the USA.
Gonorrhea is a sexually transmitted disease caused by infection with the Neisseria gonorrhoeae bacterium.
N. gonorrhea infects the mucous membranes of the reproductive tract, including the cervix, uterus, and fallopian tubes in women, and the urethra in women and men, according to the US Centers for Disease Control and Prevention (CDC).
This new study builds on a 2017 report that the outer membrane vesicle (OMV) meningococcal B vaccine, MeNZB, was reported to be associated with reduced rates of gonorrhea following a mass vaccination campaign in New Zealand.
To probe the basis for this protection we assessed cross-reactivity to N. gonorrhea of serum raised to the meningococcal vaccine Bexsero, which contains the MeNZB OMV component plus three recombinant antigens (NadA, fHBP-GNA2091, and NHBA-GNA1030).
This new information is important since gonorrhea is becoming increasingly difficult due to widespread antibiotic resistance, says the CDC.
In 2017, 555,608 cases of gonorrhea were reported to CDC.
While vaccines are routinely used for N.meningitidis, no vaccine is available for N.gonorrhea.
"We have all the knowledge and technology to make a real gonorrhea vaccine," said the producer of Bexero, GSK, in an interview during November 2018.
This study’s results may lead to human clinical trials.
These Australian researchers included Evgeny A Semchenko, Aimee Tan, ay Borrow, and Kate L Seib, Institute for Glycomics, Griffith University, Gold Coast, Australia.
Address correspondence to Kate L. Seib: k.seib@griffith.edu.au.
See the disclosures for potential conflicts of interest.
Our Trust Standards: Medical Advisory Committee
- The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae
- Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case
- Gonorrhea - CDC Fact Sheet
- Bexsero
- Glaxo considers developing Gonorrhea vaccine as threat rises