Vaccination Can Reduce Gonorrhea Cases

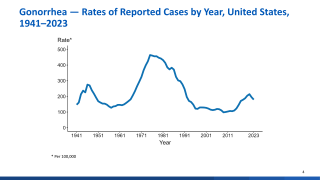
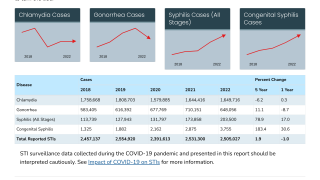
The Journal of Infection recently published insights on how vaccination can reduce the incidence of gonorrhea, a common sexually transmitted disease. Gonorrhea is one of the fastest-growing diagnoses by percent change (16.8%).
This new study's findings indicate that MenB vaccines may offer moderate protection against gonococcal infections.
Published on July 8, 2024, this systematic review and meta-analysis assessed the effectiveness of 4CMenB and MenB-OMV vaccines for meningococcal vaccination against gonococcal infections across 12 studies conducted in countries including the United States, New Zealand, Canada, Cuba, Australia, and Norway.
Modeling studies have suggested that even a partially effective vaccine could reduce the population prevalence of gonococcal infections by 35–60% within ten years and decrease incidence by up to 25% over 70 years.
These findings emphasize the potential value of incorporating meningococcal vaccination into strategies to control gonococcal infections, particularly in regions with high incidence rates and limited treatment options due to antibiotic resistance.
However, further research is needed to ascertain the factors associated with vaccine protection and to provide more substantial evidence for cost-effectiveness analysis. This will inform vaccination strategies for the more effective management of gonococcal infections.
Furthermore, other countries besides the United Kingdom should consider 4CMenB vaccination programs for those at high risk of gonococcal infection.
In the U.S., GSK's Bexsero® (MenB-4C) vaccine was U.S. FDA-approved for intramuscular injection in 2015 for 10- through 25-year-olds to prevent meningococcal group B disease caused by Neisseria meningitidis bacteria. As of 2024, people between the ages of 16 and 23 can receive the Bexsero vaccine based on 'shared clinical decision-making.'
This literature search was conducted in PubMed, Embase, Cochrane Library, CINAHL, Google Scholar, clinical trial registries, and major health and immunization conferences.
A meta-analysis was performed with the DerSimonian-Laird random-effects model to estimate the pooled VE.
All study authors receive no personal payments from the industry. The authors declare the following financial interests/personal relationships that may be considered potential competing interests: Helen Marshall is an investigator on vaccine trials sponsored by iILiAD Biotechnologies, Pfizer, and Sanofi. Helen Marshall’s institution receives funding for investigator-led studies from industry, including GSK, Pfizer, and Sanofi Pasteur.
Our Trust Standards: Medical Advisory Committee