Monkeypox Vaccine Distribution Ramps-Up
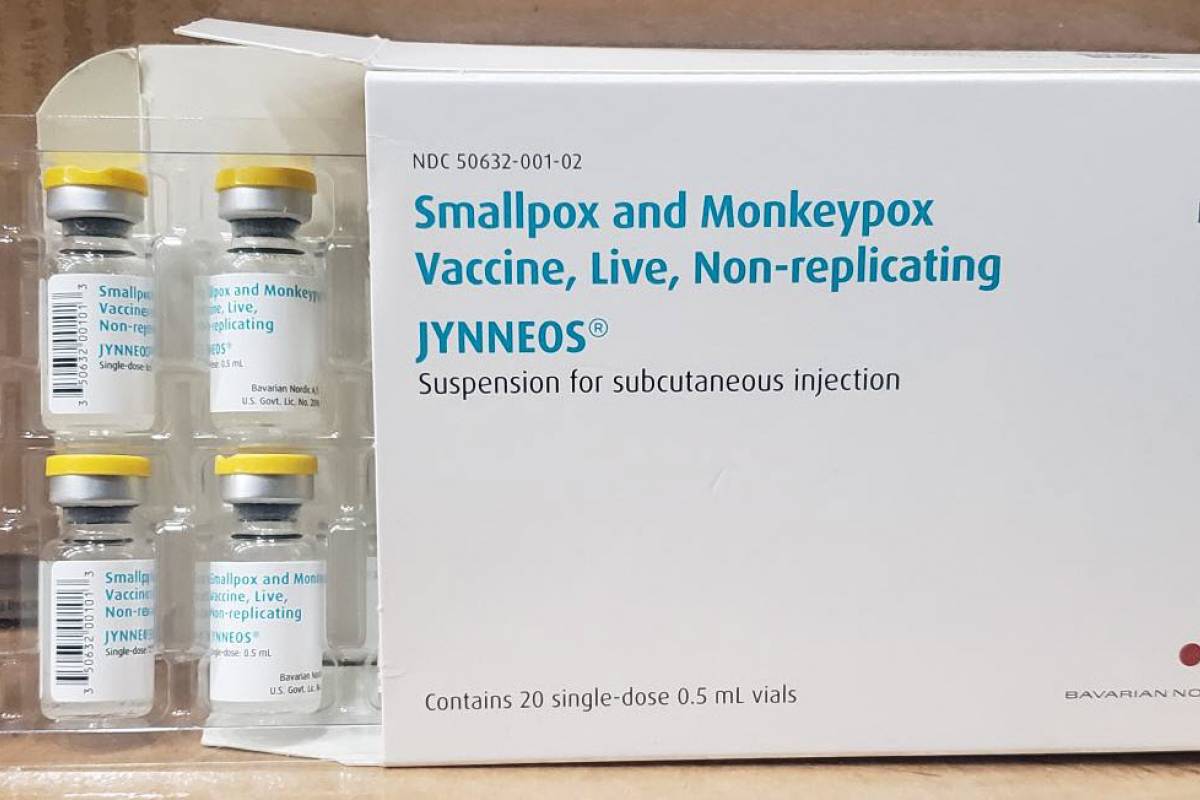
According to a recent announcement by the U.S. Department of Health and Human Services' Office of the Assistant Secretary for Preparedness and Response (ASPR), 41,250 Bavarian Nordic's JYNNEOS® vaccines were distributed in the USA on July 7, 2022.
To meet consumer demand for this U.S. FDA-licensed vaccine, ASPR announced on July 1, 2022, that it had ordered an additional 2.5 million doses of JYNNEOS vaccines to enhance monkeypox preparedness.
This new order increased the U.S. government's available supply to more than 4 million doses in the future.
On July 6, 2022, the European Medicines Agency confirmed the Emergency Task Force had recommended that the Jynneos (IMVAMUNE) vaccine could be used to protect against monkeypox in the E.U.
However, the use of JYNNEOS against monkeypox or smallpox infection is based on immunogenicity data generated in humans and animals and on protection in animals challenged with the virus.
On June 30, 2022, the U.S. CDC published 'Considerations for Monkeypox Vaccination; What You Need to Know.'
The CDC stated that 'no data are available yet on the effectiveness of these vaccines in the current monkeypox outbreak.'
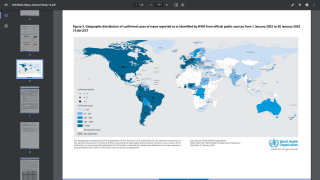
As of July 11, 2022, about (79) countries had reported about 9,860 probable monkeypox virus West African clade patients since early May 2022.
And in the USA, the CDC reported on July 11, 2022, that (39) states, the District of Columbia, and Puerto Rico had confirmed (866) monkeypox-infected patients.
Other monkeypox outbreak news is posted at Vax-Before-Travel.
Note: This information was manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee