$21.8 Million Advances Marburg Vaccine Candidate Development

The Washington DC-based Sabin Vaccine Institute today announced it is receiving an additional $21.8 million under an existing contract with the U.S. Biomedical Advanced Research and Development Authority (BARDA).
These BARDA funds will advance the development of a vaccine against Marburg virus disease.
Currently, there are no U.S. FDA-approved vaccines for Marburg disease.
The Sabin Marburg vaccine is the only candidate currently slated for a Phase 2 clinical trial.
In 2019, BARDA awarded Sabin a multi-year contract valued at $128 million to further the development of vaccines against Marburg and Ebola Sudan.
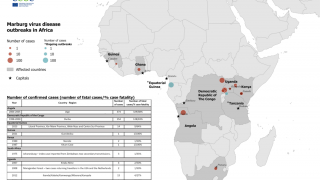
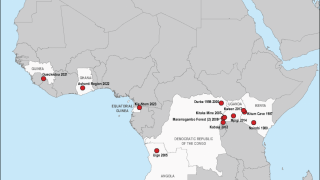
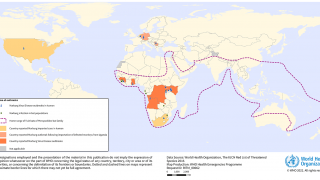
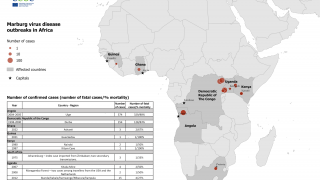
Marburg, a virus related to Ebola Zaire, is among the world's deadliest viruses, resulting in the death of approximately half the people the virus infects.
The latest BARDA funds enable Sabin to conduct a randomized, blinded, placebo-controlled clinical trial among adults in the USA and advance non-clinical vaccine dosing studies.
The Phase 2 clinical trial in the USA will begin after Sabin has initiated a same-stage trial in Africa, currently scheduled for 2023.
"Beginning Phase 2 clinical trials for the Marburg vaccine is a pivotal milestone for us, and we appreciate BARDA's continued confidence in our work and support for this critical next step," says Sabin's CEO Amy Finan, in a press release issued on September 13, 2022.
"Vaccines remain our best bet against death and disability from deadly viruses."
"I am hopeful that in the years ahead, we can offer this life-saving vaccine to every person who needs it."
Ebola Sudan and Marburg are members of the filovirus family, transmitted to humans by infected animals, particularly fruit bats.
Both viruses can cause severe hemorrhagic fever in humans.
No treatment of the hemorrhagic fevers caused by filoviruses has been licensed to date.
Once a human is infected, the virus can spread to others through close personal contact or contact with bodily fluids.
Unlike Ebola Sudan, Ebola Zaire vaccines are currently available based on clinical need.
This announcement was manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee