Novel Rabies Vaccine Candidate Conducts Phase 3 Clinical Trial
YS Biopharma Co., Ltd. today announced its PIKA Rabies Vaccine candidate received Phase 3 clinical trial approval from the Drug Regulatory Authority of Pakistan.
The PIKA Rabies Vaccine has the potential to become the first accelerated three-visit one-week regimen, superior to the currently available vaccine with a five-visit one-month or three-visit three-week regimen.
Dr. Zenaida Mojares, Chief Medical Officer of YS Biopharma, commented in a press release on May 16, 2023, "Our progress enables us to advance towards our mission of providing innovative and efficacious vaccines in the fight against a vaccine-preventable rabies disease, with almost 100% case fatality rate."
Rabies is a zoonotic infection that is a vaccine-preventable viral disease. Unfortunately, almost 60,000 people worldwide die from rabies each year.
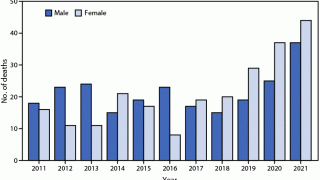
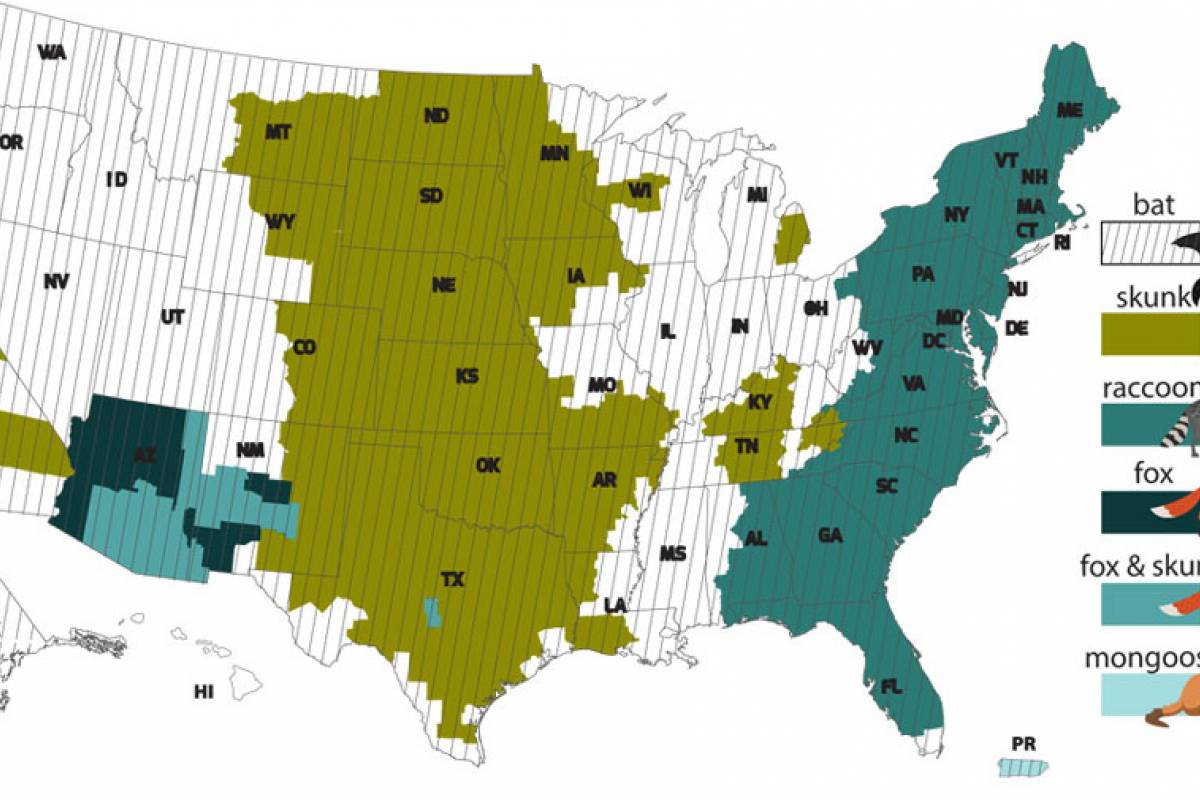
The number of human rabies deaths in the United States has been steadily declining since the 1970s thanks to animal control (bats and dogs) and vaccination programs, says the U.S. CDC.
Up to 95% of human deaths occur in Africa and Asia, where dog rabies is poorly controlled, says the WHO.
The Company has completed Phase 1 and 2 clinical trials of its PIKA Rabies Vaccine in Singapore. Another Phase 1 trial was also conducted in China. All three trials have shown that the PIKA rabies vaccine is safe, tolerable, and immunogenic.
In the U.S., various rabies vaccines are available at clinics and community pharmacies.
Note: Starting February 1, 2023, the temporary suspension for dogs entering the U.S. from high-risk countries for dog rabies was extended. This includes dogs arriving from countries without a high risk of rabies if the dogs have been in a high-risk country in the past six months.
Our Trust Standards: Medical Advisory Committee