Dual Ebola and Yellow Fever Vaccine Offers Advantages

The journal NPJ Vaccines recently reported that researchers have developed and characterized a novel dual-target single-shot vaccine candidate that protects against Ebola (EBOV) and Yellow Fever (YFV) infection.
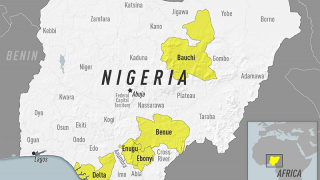
Announced on July 11, 2023, the YF-EBO pre-clinical vaccine candidate could help communities combat simultaneous EBOV and YFV epidemics, such as in Africa.
While there are approved vaccines for Ebola (Ervebo®) and Yellow fever (Stamaril®) in 2023, a single combo vaccine could enhance vaccination campaigns.
EBOV is a member of the Filoviridae family that causes severe and acute systemic disease in humans, known as Ebola virus disease, with mortality rates up to 80%.
YFV is a mosquito-borne flavivirus causing severe hemorrhagic disease in humans.
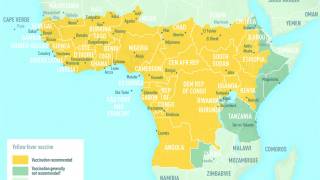
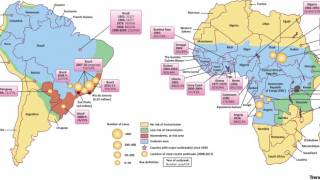
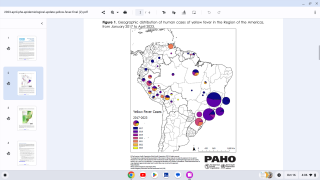
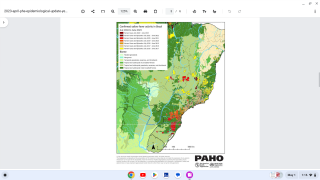
Yellow fever is endemic in Central and South America, as well as sub-Saharan Africa, where EVD surges.
Despite the availability of a very efficient live-attenuated yellow fever vaccine (Stamaril), annually, an estimated 51,000–380,000 severe cases of YF still occur, resulting in 19,000–180,000 deaths.
The re-emergence of YF outbreaks can be mainly attributed to low vaccine coverage due to supply issues.
Therefore, alike for EVD, a second-generation YFV vaccine with a sustainable supply could deliver measurable benefits during dual outbreaks.
Future studies must address whether this observed cross-reactive humoral immunity is sufficient to also provide cross-protection against heterologous challenge, ideally in step-up models using original filoviruses under BSL4 conditions, wrote these researchers.
Our Trust Standards: Medical Advisory Committee