U.S. Buys $32 Million More Japanese Encephalitis Vaccines

A specialty vaccine company today announced the signing of a $32 million contract with the United States (U.S.) Department of Defense (DoD) for the immediate supply of its Japanese encephalitis (JE) vaccine, IXIARO®.
Under this new one-year contract with Valneva SE, the DoD can purchase additional doses for the next twelve months.
IXIARO® is the only JE vaccine approved for adults by the U.S. Food and Drug Administration (FDA).
Dipal Patel, Chief Commercial Officer of Valneva, commented in a press release on September 25, 2023, "We are excited to continue our long-term relationship with the DoD."
"The U.S. military has trusted IXIARO® for over ten years to help protect military personnel, their families, civilian government service personnel, and government contractors from this potentially deadly disease."
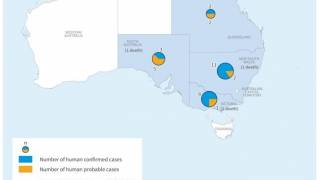
Japanese encephalitis is a deadly infectious disease found mainly in Asia. The disease is endemic in Southeast Asia, India, and China, a region with more than three billion population.
About 70,000 cases of JE are estimated to occur in Asia each year.
JE is fatal in approximately 30% of those who show symptoms, leaving half of the survivors with permanent brain damage.
In the U.S., Valneva markets and distributes IXIARO® directly to the military and private travel market.
IXIARO / JESPECT® is licensed for adults in Australia, New Zealand, Europe, Canada, Switzerland, Hong Kong, Singapore, Israel, Norway, Liechtenstein, Iceland, Singapore, Japan, the United Kingdom, and the Republic of Korea. In all other licensed territories, IXIARO/JESPECT is indicated for use by adults aged 18 years or older.
Our Trust Standards: Medical Advisory Committee