U.S. FDA Issued MedWatch Safety Alert for COVID-19 Vaccines for Children

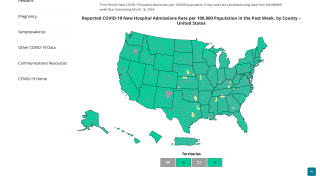
The U.S. Food and Drug Administration (FDA) MedWatch Safety Alert recently published an advisory on X informing healthcare providers who administer the Moderna COVID-19 vaccine (SpikeVax® XBB.1.5) (2023-2024 Formula) to individuals six months through 11 years of age to ensure that the correct volume of the vaccine (0.25 mL) is withdrawn from the vial and that the correct dose is administered to the vaccine recipient.
On November 1, 2023, the FDA confirmed that it had become aware that some healthcare providers may not recognize that the single-dose vial of Moderna COVID-19 Vaccine (2023-2024 Formula) for use in individuals six months through 11 years of age contains notably more than 0.25 mL of the vaccine.
Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.
The Dosage and Administration section of the Fact Sheet for Healthcare Providers Administering Vaccine has been revised to clarify that 0.25 mL should be withdrawn from the vial, and the vial and any excess volume should be discarded.
If healthcare providers, parents, or caregivers have questions, they may contact the FDA's Center for Biologics Evaluation and Research at [email protected].
On November 2, 2023, Moderna, Inc. reported financial results and provided business updates for the third quarter of 2023, but these did not highlight this MedWatch advisory.
"Through this quarter, we demonstrated our ability to increase our share in the U.S. market (Spikevax's U.S. market share to date increased to 45% from 36% in 2022). We now expect this year's vaccination rate to be similar to last fall," commented Stéphane Bancel, Moderna's CEO, in a press release.
"In the third quarter, we significantly resized our manufacturing infrastructure to make our COVID-19 franchise profitable for 2024 and beyond.
The Company reported $1.8 billion in Spikevax® vaccine sales in the third quarter of 2023. This led to $3.9 billion in vaccine sales for the year through the third quarter.
Moderna believes that the U.S. market for fall 2023 will be at least 50 million doses, supporting total 2023 Spikevax sales of at least $6 billion.
Our Trust Standards: Medical Advisory Committee