170,000 Bladder Cancer Vaccine Doses Prepared by ImmunityBio

ImmunityBio, Inc. announced today that the drug substance had been completed and successfully qualified for “fill finish” (filling vials and finishing packaging), sufficient for 170,000 doses of 400mcg ANKTIVA®.
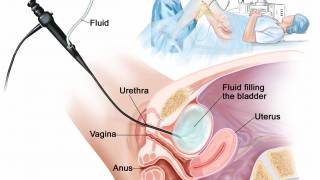
ANKTIVA (nogapendekin alfa inbakicept-pmln) is the first U.S. FDA-approved immunotherapy for non-muscle invasive bladder cancer that activates natural killer cells, T cells and memory T cells for a long-duration response.
Coupled with the recent announcement of a partnership with the Serum Institute of India for enhanced BCG vaccine availability, this provides the Company with a significant initial supply of ANKTIVA for commercial and clinical trial use in advance of the full operation of the Company’s drug substance and fill-finish manufacturing plants in California and New York.
In 2024, Anktiva will be priced at $35,800 per dose. The cost of the BCG vaccine is additional.
Since the Company’s merger with NantKwest in 2021, ImmunityBio has made significant capital investments in personnel, plants, and equipment to ensure the global capacity of the ANKTIVA drug product for both the commercial launch and clinical trials in bladder cancer and other tumor types in its pipeline.
Both drug substance and drug product facilities are nearing completion to ensure sufficient capacity and multiple GMP manufacturing sites for ANKTIVA in its approved indication and for clinical trials and future indications.
“Our belief in the importance of this molecule and its potential to evolve immunotherapy to the next level guided our strategic plan to invest for the future with anticipation of ANKTIVA’s approval,” said Rich Adcock, CEO & President ImmunityBio, in a press release on May 7, 2024.
“I’m grateful for our employees and our investors who have supported and believed in our commitment to invest for our long-term vision and future.”
The Company is applying its science and platforms to treating cancers, including developing potential cancer vaccines, immunotherapies, and cell therapies that we believe will sharply reduce or eliminate the need for standard high-dose chemotherapy.
TCompany says these platforms and their associated product candidates are designed to be more effective, accessible, and easily administered than current standards of care in oncology and infectious diseases.
Our Trust Standards: Medical Advisory Committee