Which Infants Need RSV Passive Immunization
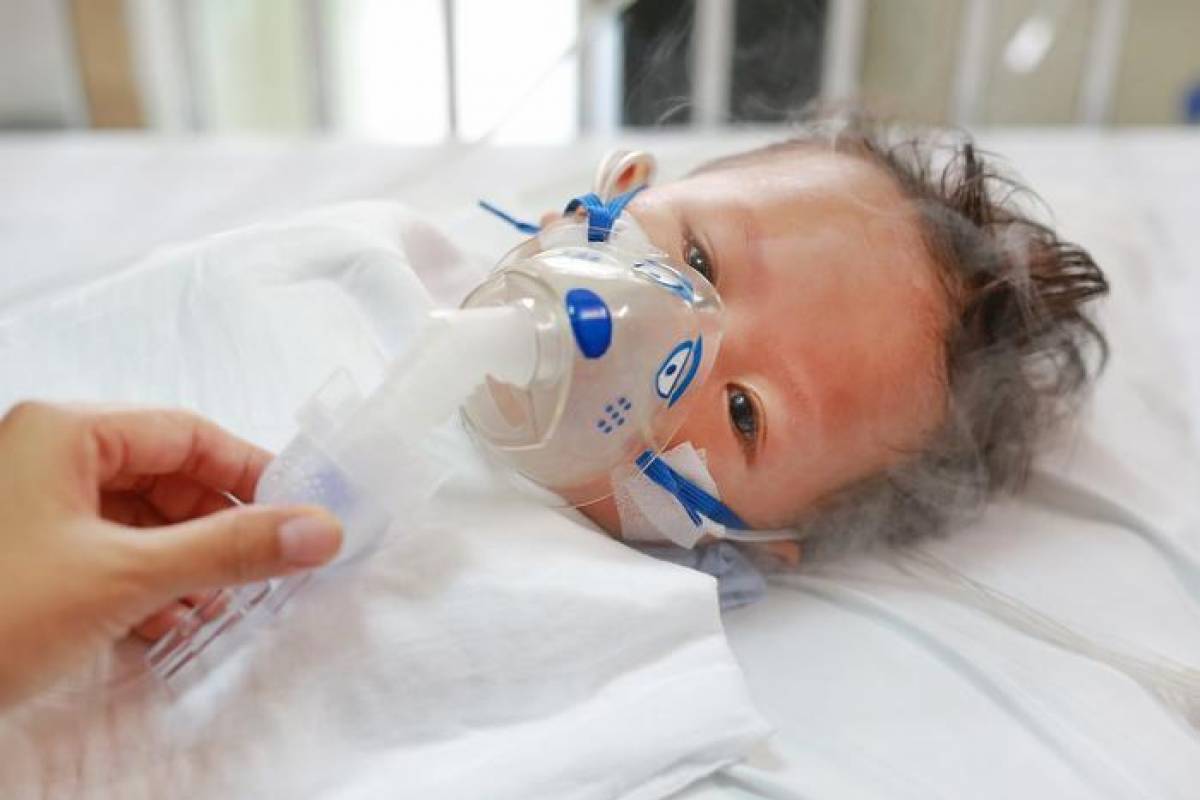
Following the initial shortage of the newly approved long-acting, monoclonal antibody Beyfortus™ (nirsevimab) for preventing infant respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI), a new digital app may help identify newborns at the highest risk for developing serious RSV LRTI, according to research published at the ATS 2024 International Conference.
These researchers wrote, 'To predict whether these infants developed severe RSV LRTI requiring ICU admission during the first year of life, we developed a multivariable logistic regression model. The model includes demographic and clinical variables collected at or shortly after birth–19 variables, such as prenatal smoking, delivery method, maternal age, and assisted breathing (ventilation) during birth hospitalization.'
"Timely identification of infants at highest risk of RSV-related morbidity is key to prevention," said lead author Brittney M. Snyder, PhD, assistant professor, Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, in a press release on May 21, 2024.
"Our personalized risk prediction tool may have applications in allocating expensive and/or limited immunoprophylaxis (immunization with nirsevimab or palivizumab) to achieve the greatest benefit and promote RSV prevention among families with high-risk infants."
During the 2023-2024 RSV peak season in the U.S., Beyfortus reduced RSV hospitalizations by about 82% in infants compared to infants who received no passive immunization against RSV.
As of March 2024, among females with an infant <8 months, 41.3% reported that their infant received Beyfortus.
As of May 2024, access to Beyfortus is not constrained in the U.S.
Our Trust Standards: Medical Advisory Committee























