RSV Protection Options Are Plentiful in 2024

For the first time, ample supplies of protective immunizations are available as the 2024-2025 Respiratory Syncytial Virus (RSV) season begins in the United States.
The Lancet Infectious Diseases recently published an overview of RSV immunizations, highlighting different target populations, antigens, and clinical trial results.
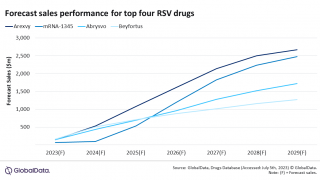
This overview, published on September 23, 2024, discussed the market approval of a protein-based maternal vaccine and monoclonal antibody (mAb) to protect infants. About 40% of infants were administered the mAb Beyfortus (nirsevimab) last RSV season.
First-year experience protecting infants with Beyfortus in high-income countries such as the U.S. shows a significant public health benefit.
On July 10, 2024, the New England Journal of Medicine reported that in a real-world setting, Beyfortus reduced the risk of infant hospitalization for RSV- associated bronchiolitis by 83%.
There are also mRNA and two protein-based RSV vaccines approved for older adults.
These researchers wrote, 'It is expected that the RSV vaccine landscape will continue to develop to protect all people globally in the coming years.'
'The vaccine and mAb landscape remain active with 30 candidates in clinical development using four approaches: protein-based, live-attenuated and chimeric vector, mRNA, and mAbs.'
'Candidates in late-phase trials aim to protect young infants using mAbs, older infants and toddlers with live-attenuated vaccines, and children and adults using protein-based and mRNA vaccines.'
'As RSV vaccines have not yet reached low-income and middle-income countries, we outline urgent steps to minimize the vaccine delay.'
These RSV immunization products are generally available at health clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee