Saudi Arabia Camels Continue Spreading Deadly MERS

The year 2024 was named "The Year of The Camel" to celebrate the unique cultural value represented by the inhabitants of the Arabian Peninsula. Today, camels remain a living witness of authenticity and a core cultural component of Saudi identity.
However, camels are also the source of a deadly virus.
A recent study found the Middle East respiratory syndrome coronavirus (MERS-CoV) spreads among dromedary camels in the Arabian Peninsula. Still, the reasons for this are not well understood.
The virus is mainly transmitted to people through direct contact with infected camels.
On September 14, 2024, the researchers stated that the ongoing spread of MERS-CoV from camels to humans raises significant public health concerns.
Because MERS-CoV is transmitted from animals to humans, understanding how the virus evolves is crucial for addressing human disease. Efforts to control the impact of MERS-CoV on human populations are being challenged by lineage B5 viruses, which have improved abilities to replicate and spread among dromedary camels.
This new preprint study found of 558 dromedary camel nasal swabs from Saudi Arabia sampled from November 2023 to January 2024, 39% were positive for MERS-CoV RNA.
This finding indicates people should be cautious when engaging with camels in Saudi Arabia.
Furthermore, MERS infections can be very harmful to humans.
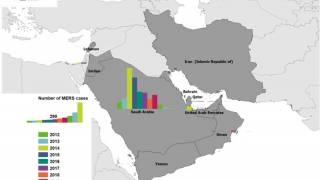
Since April 2012, and as of early August 2024, the European CDC reported a total of 2,622 cases of MERS, including 953 deaths (36%), have been reported worldwide. This year, four MERS fatalities have been reported in sections of Saudi Arabia.
From a protection perspective, the World Health Organization says several MERS vaccine candidates are conducting human clinical trials in 2024. Efforts to develop an effective and safe human MERS vaccine have progressed as of September 22, 2024, with a few vaccine candidates reaching human studies.
For example, CEPI announced funding up to $34.8 million to develop and stockpile the VTP-500 (ChAdOx1 MERS) vaccine. Oxford University's Pandemic Sciences Institute and Barinthus Biotherapeutics Inc. are the developers of this vaccine candidate.
In preparation for an MERS outbreak, the European Medicines Agency has confirmed support for the program through the PRIME designation.
In a related press release, Bill Enright, Barinthus Bio's chief executive officer, stated, "We are thrilled to be working with the University of Oxford and CEPI on developing this critical vaccine candidate. "
"There is an active need for a MERS vaccine for at-risk populations and travelers in the Middle East. It is critical to ensure we have the necessary countermeasures to protect people worldwide from deadly pathogens such as MERS, which have the potential for future outbreaks."
As of September 2024, the U.S. CDC has not issued a travel advisory regarding the MERS cases in Saudi Arabia.
Our Trust Standards: Medical Advisory Committee